Article Text
Abstract
Introduction Type 2 diabetes (T2D) is one of the most frequent comorbid medical conditions in pregnancy. Glycaemic control decreases the risk of adverse pregnancy outcomes for the pregnant individual and infant. Achieving glycaemic control can be challenging for Medicaid-insured pregnant individuals who experience a high burden of unmet social needs. Multifaceted provider–patient-based approaches are needed to improve glycaemic control in this high-risk pregnant population. Mobile health (mHealth) applications (app), provider dashboards, continuous glucose monitoring (CGM) and addressing social needs have been independently associated with improved glycaemic control in non-pregnant individuals living with diabetes. The combined effect of these interventions on glycaemic control among pregnant individuals with T2D remains to be evaluated.
Methods and analysis In a two-arm randomised controlled trial, we will examine the combined effects of a multicomponent provider–patient intervention, including a patient mHealth app, provider dashboard, CGM, a community health worker to address non-medical health-related social needs and team-based care versus the current standard of diabetes and prenatal care. We will recruit 124 Medicaid-insured pregnant individuals living with T2D, who are ≤20 weeks of gestation with poor glycaemic control measured as a haemoglobin A1c ≥ 6.5% assessed within 12 weeks of trial randomisation or within 12 weeks of enrolling in prenatal care from an integrated diabetes and prenatal care programme at a tertiary care academic health system located in the Midwestern USA. We will measure how many individuals achieve the primary outcome of glycaemic control measured as an A1c<6.5% by the time of delivery, and secondarily, adverse pregnancy outcomes; patient-reported outcomes (eg, health and technology engagement, literacy and comprehension; provider–patient communication; diabetes self-efficacy; distress, knowledge and beliefs; social needs referrals and utilisation; medication adherence) and CGM measures of glycaemic control (in the intervention group).
Ethics and dissemination The Institutional Review Board at The Ohio State University approved this study (IRB: 2022H0399; date: 3 June 2023). We plan to submit manuscripts describing the user-designed methods and will submit the results of the trial for publication in peer-reviewed journals and presentations at international scientific meetings.
Trial registration number NCT05662462
- Diabetes in pregnancy
- OBSTETRICS
- General diabetes
- Clinical Trial
- Health Equity
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
ACHIEVE is a multicomponent intervention using a Mobile health (mHealth) application (app), provider dashboard, continuous glucose monitoring, referral for social needs and team-based care to address glycaemic control.
Strengths of the ACHIEVE trial include an intervention focused on Medicaid-insured individuals with type 2 diabetes and poor glycaemic control who are at high risk of adverse pregnancy outcomes.
Limitations of the ACHIEVE trial include that it is powered for pregnancy glycaemic control and not adverse pregnancy outcomes and periconception or postpartum outcomes.
Challenges of this trial will include engaging a high-risk population of pregnant individuals with poor glycaemic control and unmet social needs using a mHealth intervention.
Introduction
Background
Type 2 diabetes (T2D) is one of the most frequent chronic comorbid conditions in pregnancy.1–3 Every year in the USA more than 100 000 pregnancies are complicated by T2D, which is anticipated to double in the next 10 years, affecting 1 in 20 pregnancies.4–6 T2D increases the risk of adverse pregnancy outcomes for the pregnant individual, including severe maternal morbidity, caesarean delivery, preeclampsia and severe maternal morbidity, and infant, including large for gestational age at birth, preterm birth and neonatal hypoglycaemia.7–9 Inadequate glycaemic control further increases the risk of these adverse outcomes by at least two-fold.10–12
Improving glycaemic control as measured by haemoglobin A1c decreases the risk of adverse pregnancy outcomes.7 12 Guidelines recommend achieving an A1c target in pregnancy of at least <6.5% to optimise outcomes.13 14 Glycaemic control for pregnant individuals with T2D can be achieved with insulin pharmacotherapy, consistent glucose monitoring, lifestyle modifications including diet and exercise and interdisciplinary, team-based diabetes and prenatal care.11 Pregnant individuals living with T2D who experience a higher burden of adverse social determinants of health (SDoH) and unmet non-medical health-related social needs (social needs),10 including food insecurity15 and inadequate physical activity,16 are less likely to achieve glycaemic control.
More than half of pregnant individuals with T2D are insured by Medicaid.17 Medicaid-insured pregnant individuals experience a higher burden of T2D, adverse pregnancy outcomes and adverse SDoH.18–20 Addressing modifiable social needs that affect glycaemic control in this population could improve pregnancy outcomes by addressing maternal health inequity.10 21–25 A model that addresses unmet social needs for individuals with chronic comorbidities is the Agency for Healthcare Research and Quality Pathways Community Hub model.26 The Hub model leverages care coordination with community health workers (CHWs) to facilitate social need-related screening and referrals through the use of social needs pathways.27
Mobile health (mHealth) applications (app) can address health disparities in prenatal access and care due to adverse SDoH.28 29 mHealth apps are pervasive among reproductive-age female individuals, and >90% actively engage in their use.30 mHealth apps include access to digital information, reminders and convenient communication modes with healthcare providers. Electronic data linked to patients’ mHealth apps can allow the care team to actively engage with patients in their care and help them to achieve treatment goals.31 32 In addition, continuous glucose monitoring (CGM) is a personal mobile technology that allows identification of precise, individualised patterns of dysglycaemia.33 CGM has been shown to improve glycaemic control and decrease the risk of adverse pregnancy outcomes for individuals with type 1 diabetes,34 but its impact among pregnant individuals with T2D remains largely unstudied.33
Current mHealth apps for diabetes management typically lack user-centred design (UCD) features and are not holistically focused on the combination of addressing unmet pregnancy and social needs, CGM integration and team-based care supported with provider dashboards. Whether an integrated, theory-driven and user-centred intervention can result in improved glycaemic control and patient-reported outcomes (PROs) among Medicaid-insured pregnant individuals with T2D remains to be answered.
The proposed ACHIEVE intervention integrates new and existing technologies to develop an innovative ecosystem, including a mHealth patient app, provider dashboard, CGM device, social needs referrals and team-based care.
Aim and hypotheses
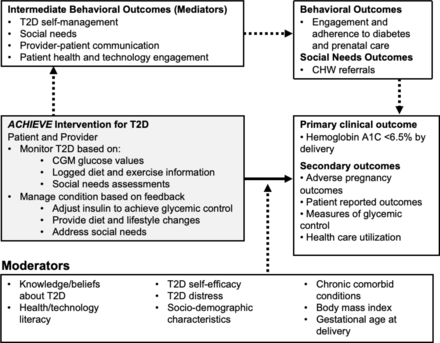
The objective of ACHIEVE is to test whether a multicomponent intervention (mHealth app, provider dashboard, CGM device, social needs referrals and team-based care) versus the current standard of care for prenatal and diabetes care results in improved glycaemic control by the time of delivery (A1c <6.5%) for Medicaid-insured pregnant individuals with T2D (figure 1).
ACHIEVE conceptual framework. CHW, community health worker; T2D, type 2 diabetes.
Primary hypothesis
We hypothesise a 25% absolute increase in the proportion of individuals in the intervention group who will meet the target A1c <6.5% by the time of delivery compared with the standard care group.
Secondary hypotheses
We hypothesise that individuals in the intervention group will have fewer adverse pregnancy outcomes and superior PROs (ie, health and technology engagement, literacy and comprehension; provider–patient communication, diabetes self-management, self-efficacy, distress, knowledge and beliefs; social needs referrals and utilisation and medication adherence) compared with the standard care group.
Methods and analysis
Design
ACHIEVE is a randomised, controlled, single-centre superiority trial to determine whether Medicaid-insured pregnant individuals with T2D enrolled in a multicomponent intervention (mHealth app, provider dashboard, CGM device, social needs referrals and team-based care) will improve glycaemic control compared with those receiving standard prenatal and diabetes care (figure 2). Each subcomponent of the proposed intervention is grounded in social cognitive theory (SCT) and aims to address an individual’s skills, knowledge and beliefs and self-efficacy to achieve glycaemic control.35 Data management and coordination will occur by an independent team at the clinical site of the ACHIEVE trial and will be led by the study statistician (XP). Participant data will be collected, stored and maintained Research Electronic Data Capture (REDCap), a secure and confidential data management system. Randomisation by computer-generated random numbers by the study statistician will be performed with a 1:1 non-blinded allocation between intervention:standard care groups. The allocation will be kept in a sealed envelope until the research staff enrols and assigns a participant to a study group. An independent data safety monitoring board will monitor and audit trial conduct, including review of any adverse events, annually during the clinical trial.
ACHIEVE intervention components. CGM, continuous glucose monitoring; EHR, electronic health record; PRO, patient reported outcome; SCT, Social Cognitive theory; T2D, type 2 diabetes.
Population
Individuals will be recruited as part of The Ohio State University Diabetes during Pregnancy programme, which provides integrated diabetes and prenatal care at a tertiary academic medical centre located in the Midwestern USA.12 36 Some individuals continue to receive routine prenatal care in their local community and then receive high-risk prenatal and diabetes care from the programme. These individuals will also be eligible for study participation. The ACHIEVE trial will end at delivery, and postpartum individuals in both the intervention and standard care group will be provided with a referral to an endocrinologist and primary care provider for postpartum diabetes and reproductive healthcare.
Inclusion criteria
Individuals with an ultrasound-confirmed intrauterine pregnancy, aged ≥18 years, with a diagnosis of T2D, with poor glycaemic control measured as an A1c ≥6.5% assessed within 12 weeks of trial randomisation or within 12 weeks of enrolling in prenatal care, who are ≤20 weeks of gestation at trial randomisation, English or Spanish speaking, who are Medicaid insured or Medicaid eligible, and available to participate in a longitudinal study across pregnancy will be eligible. Because this intervention includes an mHealth app, individuals will need to use a smartphone (either iPhone or Android) with internet access. For those without a smartphone, a device and access to the internet will be provided for the duration of the trial.
Exclusion criteria
We will exclude individuals who cannot cognitively complete the study requirements, consent to all study activities through the time of delivery, be accessible for participation in study activities or cannot read and write in either English or Spanish. Individuals enrolled in a concurrent clinical trial focused on improving glycaemic control will not be eligible for enrolment.
Recruitment
Individuals will be recruited over a 3-year time period. Potentially eligible individuals will be identified via the electronic health record (EHR). In addition, individuals will be recruited using flyers placed in clinic and direct provider referrals from the community.
Baseline visit
A research assistant or healthcare provider will ask patients whether they are interested in joining the study and they will be referred for an orientation visit. Informed consent will be obtained in person in English or Spanish. Baseline data, including demographics, diabetes history and information about current technology familiarity and utilisation will be collected using electronic data capture. Participants will complete a blood draw for measurement of A1c using a standardised assay. Enrolled individuals will be randomised in a 1:1 ratio to the intervention: standard care. Participants randomised to the intervention group will: (1) receive assistance in downloading and using the mHealth app via their smartphone and using the CGM device for glucose monitoring and (2) be trained with placing DEXCOM G7 CGM sensors and transmitters including instruction about frequent troubleshooting with new CGM initiation.
Partial patient and public involvement
Medicaid-insured pregnant individuals with T2D and their healthcare providers, including both physicians and nurses, clinical social workers and CHWs, have collaboratively worked with the study team in designing the mHealth app and dashboard interface by providing feedback about the intervention prototype via structured interviews over multiple iterations.31 Additional testing of the integrated system and tools (ie, patient mHealth app and provider dashboard) to address usability and patient/provider centeredness will be conducted prior to the randomised controlled trial (RCT).
Measures
The primary clinical outcome is the proportion of individuals with an A1c <6.5% by the time of delivery. For those individuals with more than one A1c assessment in late pregnancy, the value most proximate to delivery will be used as a measure of the risk of adverse pregnancy outcomes at birth associated with dysglycaemia.12 For most participants without a preterm delivery, this A1c assessment will be in the late third trimester. A1c will be assessed once per trimester, consistent with US guidelines for diabetes management in pregnancy.13 14 Of note, we selected an A1c threshold of <6.5% as opposed to <6.0% as an aggressive target, <6.0% may result in more frequent episodes of hypoglycaemia, and the risk of adverse neonatal outcomes are similar at both thresholds.7 12 A1c will be measured using automated HPLC (VARIANT II TURBO HgbA1c Kit, Bio-Rad Laboratories, Hercules, California).
Search Result
Secondary adverse pregnancy outcomes at birth related to pregnancy dysglycaemia include large-for-gestational-age at birth (standardised birthweight >90% for gestational age) using a recent US infant sex-specific standard,37 neonatal hypoglycaemia (defined as any blood glucose <30 mg/dL during the delivery admission), hypertensive disorder of pregnancy,38 neonatal intensive care unit admission (NICU) admission for any indication, preterm birth <37 weeks per the best obstetric estimate for any indication39 and respiratory distress syndrome.40
Secondary measures of glycaemic control for the standard care group will include: weekly self-monitored blood glucose values, including both fasting and 2-hour postprandial values, at guideline-recommended target values and for the intervention group using CGM will include: the percentage of time in range between 63 mg/dL and 140 mg/dL, consistent with emerging data for diabetes in pregnancy34 as well as both continuous and dichotomous measures (≥85%). We will also assess CGM summary statistical indices including mean CGM glucose levels during the day and night, area under the curve, time spent above and below target range and low and high blood glucose indices.34
PROs will be collected at prespecified intervals at randomisation, during pregnancy and at delivery, including patient knowledge, skills and confidence to manage health using the Patient Activation Measure,41 social needs using the Accountable Health Communities Health-Related Social Needs Screening Tool,42 provider–patient communication using the Doctor-Patient Communication Scale43 and health literacy using the Short Assessment of Health Literacy-Spanish and English and eHealth Literacy Scale.44 45 In addition, we will assess diabetes management-related PROs including Diabetes Knowledge Questionnaire,46 Diabetes Distress Scale,47 Diabetes Management Self-Efficacy Scale48 and the Morisky Medication Adherence Scale.49
Healthcare utilisation will be abstracted from the EHR and patient surveys and will include prenatal visits, antepartum hospitalisations, emergency department visits, obstetric triage visits, and unscheduled clinic visits.
Within the intervention group, engagement data will be collected through log files and will be measured as the number of times a participant uses the mHealth app (total and average use of the app and specific functions) as well as the number of social needs referrals made and completed. For participants who are lost to follow-up or discontinue participation, clinical outcomes will be abstracted from the EHR, and PROs will be assessed until trial discontinuation.
Procedures
UCD testing
A UCD Work Group (UCDWG) comprised of 10 individuals and representing key stakeholder groups, including physicians (maternal–fetal medicine and endocrinology), certified diabetes care and education specialists, nurses, Medicaid-insured pregnant individuals living with T2D, CHWs, and licensed clinical social workers will guide the adaptation and refinement of the intervention prior to participant enrolment through multiple iterations of testing. The UCDWG will provide input on the mHealth app and provider dashboard functions.
Interventions
The ACHIEVE intervention is supported by a robust digital electronic platform. This platform is integrated with REDCap and other electronic systems, including the ACHIEVE mHealth app and provider dashboard, HUB Care Coordination System portal and EHR data. REDCap is a secure, web-based application designed to support data capture for research studies, including data entry, audit trails for tracking data manipulation, automated export procedures and procedures for importing data from external sources.50 The ACHIEVE intervention can tailor care in real time based on changing medical and social needs. It uses rule-based algorithms to synthesise data reported by participants and providers and adjusts the collection of PROs based on defined parameters and providing personalised educational content to patients via the mHealth app.
Participants will receive training with regards to using the mHealth app and CGM from certified diabetes care and education specialists. Participants will be further supported to engage in care pathways to resolve unmet social needs with the assistance of CHWs. Secure messaging will allow for electronic communication between participants and the certified diabetes care and education specialists and CHWs.
We describe each of the key elements of the ACHIEVE intervention below, namely, the patient mHealth app, provider dashboard, CGM device, social needs referrals and team-based care (figure 2).
mHealth application
The mHealth app provides education, reminders, care goals, care pathway recommendations, summaries of CGM data and PROs, messaging and a calendar function for goal tracking. Content is based on current US clinical guidelines for T2D in pregnancy.13 14 The mHealth app directs participants to online diabetes and pregnancy resources and learning. Assessment of patient-reported outcomes will be embedded in the mHealth app, and rule-based algorithms will provide tailored goals for medical and social needs and information on how to achieve them (ie, care pathways) and establish the frequency of assessment for PROs including social needs screening. Data are transferred to the digital health platform, which postprocessing displays data on the provider dashboard.
Provider dashboard
The ACHIEVE intervention will include a bi-directional dashboard that displays information about participants, including priority goals and care pathways, and recommendations generated via the digital platform. The dashboard will present recommendations for participant goals and care pathways provided by the digital platform algorithms. Providers can access the dashboard embedded within an online portal to monitor participant progress and close the loop on participant tasks. Both certified diabetes care and education specialists and CHWs can assess social needs pathway selections and assess recurring needs through the dashboard. Providers can use auto-generated recommendations or manually select recommendations from a clinically validated data repository.
Continuous glucose monitoring.
Participants in the intervention group will use DEXCOM G7 CGM sensors and transmitters for glucose monitoring. The Dexcom CGM system is accurate and safe in pregnant individuals with diabetes.51 The DEXCOM G7 CGM system was recently approved by the United States Food and Drug Administration (FDA) for use in pregnancy.52 Participants will be taught how to place and remove CGM sensors and will then replace sensors themselves every 10 days. DEXCOM sensors can be applied on the abdomen, arm or upper buttocks, is well-tolerated in pregnancy, and do not require calibration.51 The mHealth app will allow for wireless synchronisation with the CGM transmitter, so that data are reported back to the healthcare team, which will be reviewed by the certified diabetes care and education specialist at least two times weekly.
Social needs referrals
We will partner with Health Impact Ohio’s Central Ohio Pathways Hub, which consists of three features: (1) the Hub, a regional coordination entity, which employs CHWs to assess the social needs of patients and connect them to community resources; (2) the CHWs initiate a ‘social need care pathway’, a defined action plan to address each patient’s unique needs, which is recorded and tracked in an electronic database and (3) completion of each pathway is linked to payment from insurance companies (eg, Medicaid-managed care plans) based on specific performance benchmarks.27 53 The CHWs are embedded within the local communities in which participants live in. Participants will be screened at enrolment and throughout the intervention for social needs using a survey adapted from the Accountable Health Communities Health-Related Social Needs Screening Tool and prior studies.54 55 Once an unmet social need is identified, participants will be referred to the Health Impact Ohio Hub through the provider dashboard to address unmet social needs (eg, food insecurity, unstable housing, unemployment). Health Impact Ohio CHWs will perform a comprehensive social needs assessment and connect the participant to community resources through Hub pathways.
Team-based care
Team-based care will be provided by physicians, nurses, certified diabetes care and education specialist, and clinical social workers as well as CHWs. Team-based care will be facilitated by the provider dashboard and patient mHealth app. The clinical care pathways will address lifestyle factors, including diabetes nutrition therapy, physical activity, smoking cessation, psychosocial stress reduction and pharmacologic management (pharmacotherapy adherence and modifications) to improve glycaemic control.
Standard care arm
Participants randomised to the standard care group will receive the current standard of diabetes and prenatal care at our centre as part of the integrated diabetes and prenatal care programme.11 12 Participants will complete weekly self-monitored blood glucose logs on paper, which will be electronically sent to the EHR for provider review. Communication with the certified diabetes care and education specialists and CHWs will be via Epic’s MyChart instance (Epic MyChart). A1c will be assessed, and PROs will be collected at pre-specified intervals similar to the intervention arm (ie, approximately monthly).
Statistical analysis plan
Baseline characteristics
We will provide descriptive summaries and examine any potential differences in sociodemographic and clinical characteristics between the intervention and standard care groups using χ2 tests for categorical variables and independent two sample t-tests for continuous variables.
Primary and secondary hypothesis
Primary analyses will follow the intention-to-treat principle in which pregnant individuals will be analysed in the group to which they were randomised, regardless of whether they receive the assigned intervention or discontinue prior to delivery. As this is a randomised trial, we will not adjust for baseline participant characteristics or participant engagement measures in the primary intention-to-treat analysis unless we identify statistically significant differences between the intervention and standard care groups. No interim analyses are planned. Multiple imputations will be considered for missing covariates.
Preplanned post hoc analyses
We will conduct subgroup analyses to determine the effect of the intervention among subgroups if there is a significant interaction effect between the subgroup of interest and the treatment effect, including self-reported race and ethnicity, body mass index at randomisation, gestational age at randomisation and baseline A1c at randomisation. Exploratory analyses will also be conducted to assess the effect of the intervention on intermediate outcomes (eg, social needs, T2D self-management, provider–patient communication) and the mediating and moderating roles of the intermediate outcomes.
Power calculations and sample size
Recent pharmacological intervention trials in pregnancies complicated by T2D have assessed medium effect sizes approximating absolute changes of 15% to 30%.56 57 We target to detect a 25% absolute increase in the proportion of participants in the intervention versus standard care group with an A1c <6.5% by delivery (64% intervention vs 39% control). A total sample size of 124 participants (62 per study arm) will provide at least 80% power to detect such a difference between the two groups after accounting for up to 10% loss-to-follow-up based on a one-sided Fisher’s exact test. The loss-to-follow-up rate of 10% is based on prior diabetes in pregnancy trials at our centre.32 33
Compensation
Participants in both study arms will receive compensation of US$100 per month for participation in the trial from randomisation to delivery for completion of study activities, including study surveys, attending study sessions and reporting PROs, including clinical and social needs outcomes.
Ethics and dissemination
The OSU Institutional Review Board (IRB) has approved this protocol. All protocol amendments will be communicated for approval to the OSU IRB. We will follow Consolidated Standards of Reporting Trials (CONSORT) guidelines. We will submit study results for publication in peer-reviewed journals and presentation at international meetings. We will attempt to publish all findings in open-access journals when possible, or in other journals with a concurrent uploading of the manuscript content into PubMed central for public access. Curated technical appendices and statistical code will be made available from the corresponding authors on request.
Discussion
In this RCT, we will examine the effect of a multicomponent intervention (mHealth app, provider dashboard, CGM device, social needs referral and team-based care) versus current standard of care for prenatal and diabetes care on glycaemic control, patient-reported outcomes and pregnancy outcomes among Medicaid-insured pregnant individuals with T2D. The ACHIEVE trial brings together multiple technologies in an integrated and theory-driven framework to facilitate comprehensive, tailored, patient-centred and team-based T2D management in pregnancy. If effective, ACHIEVE will advance health equity by addressing the pregnancy and diabetes care needs of a high-risk and underserved patient population.
Decreasing disparities in glycaemic control and adverse pregnancy outcomes through addressing social needs
ACHIEVE is an intervention to address glycaemic control and consequently adverse pregnancy outcomes and to improve the patient experience among Medicaid-insured pregnant individuals living with T2D. This is an at-risk population that experiences a high burden of adverse pregnancy outcomes and inadequate glycaemic control that are related to unmet social needs.10 While clinical care is critical, it is insufficient as social needs, such as food security, adequate housing, safe environment and access to healthcare, also impact diabetes and pregnancy outcomes.22 The ACHIEVE intervention aims to provide an integrated care ecosystem that incorporates digital communication, education and remote care management to address the structural challenges faced by Medicaid-insured pregnant individuals living with diabetes and poor glycaemic control.58
Multicomponent intervention for proactive health transformation
ACHIEVE is an integrated multicomponent intervention (mHealth app, provider dashboard, CGM, social needs referrals and team-based care), employs a SCT paradigm and is informed by prior informatics-based and behavioural-based interventions to promote glycaemic control among non-pregnant individuals with diabetes.59–61 Few existing apps for prenatal care provide comprehensive evidence-based educational content, tracking tools, UCD and the potential for integration with the EHR.62 63 For T2D in pregnancy, studies using a tailored mHealth app with CGM remain to be conducted.33 64 ACHIEVE integrates health information technology tools and a dynamic, closed loop system, so that patients and providers can track treatment goals and care pathways.
Sustainable and scalable intervention
The patient mHealth app and provider dashboard of the intervention are embedded within an existing electronic health platform and REDCap.50 In addition, the intervention enhances existing delivery systems, including the established Central Ohio Pathways HUB model to address social needs. Should efficacy of the ACHIEVE intervention be demonstrated, this programme could be deployed across healthcare systems. To assist with future transferability of the intervention across clinical sites, we plan to provide technical and implementation documentation of the ACHIEVE intervention via GitHub.
Limitations and strengths
Limitations
First, this study is non-blinded to providers and patients as they need to be aware of intervention allocation. However, study arm will be blinded to those assessing and analysing the association between the intervention and primary outcome (A1c), including both the laboratory staff and biostatistician. Second, this study is powered for a primary clinical outcome of glycaemic control that is associated with adverse pregnancy outcomes but is not specifically powered for adverse pregnancy outcomes. Should this intervention demonstrate efficacy for glycaemic control, the next step would be larger clinical trial powered to detect whether adverse pregnancy outcomes can be prevented. Third, this intervention is focused on glycaemic control during pregnancy, and periconception and postpartum glycaemic control are not primarily addressed by this intervention. In the future, this intervention could be expanded to include these critical time periods. Finally, this single-site study is restricted to Medicaid-insured pregnant individuals with T2D and poor glycaemic control. Hence, these findings may not necessarily be generalizable to all pregnant individuals with T2D.
Strengths
T2D is one of the most frequent chronic comorbid conditions in pregnancy. Medicaid-insured pregnant individuals experience a higher burden of T2D, associated adverse pregnancy outcomes and unmet social needs.18–20 We aim to develop a scalable model of care that addresses modifiable social needs that directly affect glycaemic control. If this intervention proves to be efficacious, it may have major public health impact.
Ethics statements
Patient consent for publication
References
Footnotes
KKV, JJJ and NF are joint first authors.
KKV, JJJ and NF contributed equally.
Contributors KKV, JJJ and NF designed the study. ASM and TRH provided oversight for study design and implementation. NF with support from CS and RS led the design and implementation of the user-centred design phase. NF, KKV and JJJ with support from TS, AB and LB led the RCT. XP provided statistical support and oversight. JH and CB led the assessment and implementation of social needs pathways. KKV, JJJ and NF wrote the methods manuscript. All authors revised the manuscript for relevant scientific content and approved the final version of the manuscript.
Funding This trial is funded by an R01 to Drs. Fareed, Joseph, and Venkatesh, HS028822 from the Agency for Healthcare Research and Quality. In addition, Drs. Fareed, Joseph and Venkatesh received support for this trial from the Care Innovation and Community Improvement Program at The Ohio State University and DEXCOM (Grant #IIS-2021–151). This publication was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Number UL1TR002733. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.