Suspected sepsis: summary of NICE guidance
BMJ 2016; 354 doi: https://doi.org/10.1136/bmj.i4030 (Published 11 August 2016) Cite this as: BMJ 2016;354:i4030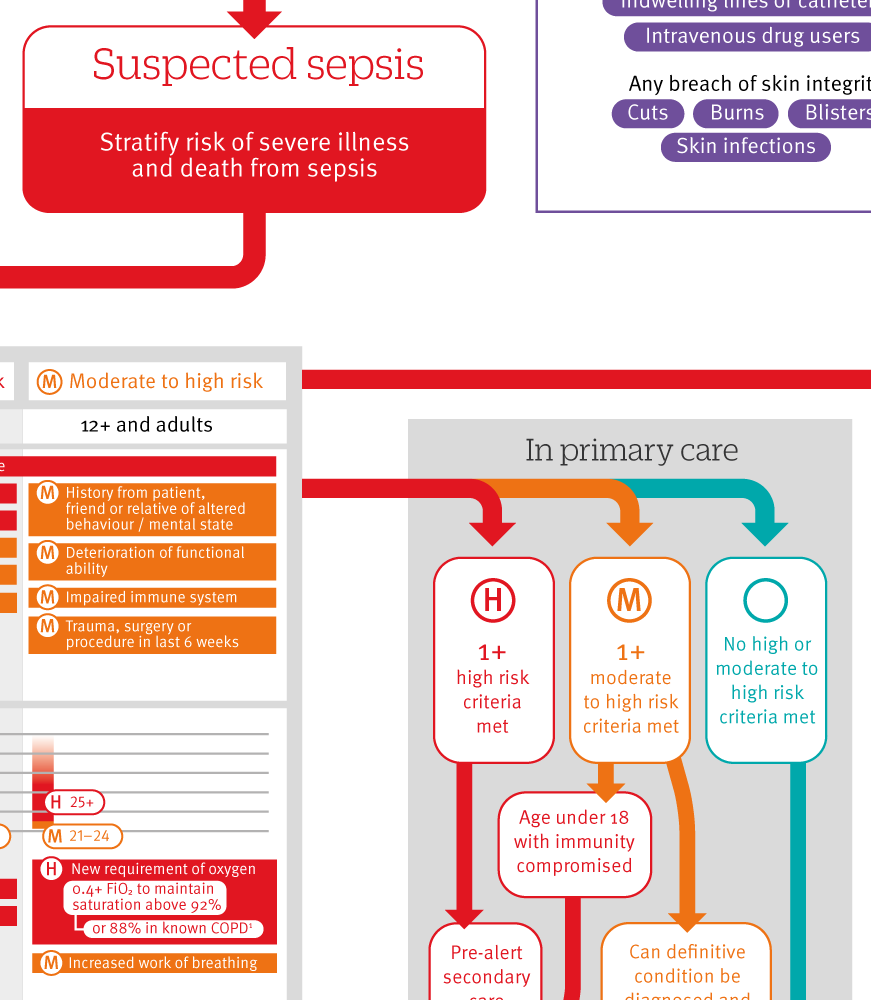
Infographic summary
Click here to see an infographic, summarising risk assessment and management of people with suspected sepsis.
- Andreas Freitag, senior research fellow1,
- Margaret Constanti, senior health economist1,
- Norma O’Flynn, clinical director1,
- Saul N Faust, professor of paediatric immunology and infectious diseases, director2
- on behalf of the Guideline Development Group
- 1National Guideline Centre, Royal College of Physicians, London NW1 4LE, UK
- 2NIHR Wellcome Trust Clinical Research Facility, Faculty of Medicine, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, UK
- Correspondence to: SN Faust, s.faust{at}soton.ac.uk
What you need to know
“Think sepsis” in any person with suspected infection
Sepsis may present with non-specific symptoms and signs and without fever
Have a high index of suspicion of sepsis in those who are aged <1 year or >75 years, pregnant, or immunocompromised, and those who have a device or line in situ or have had recent surgery
Use risk factors and any indicators of clinical concern to decide if full assessment is required
Offer people at high risk of sepsis broad spectrum antibiotics and intravenous fluids in hospital
The UK Parliamentary and Health Service Ombudsman inquiry “Time to Act” found failures in the recognition, diagnosis, and early management of those who died from sepsis,1 which triggered this guidance. In sepsis the body’s immune and coagulation systems are switched on by an infection and cause one or more body organs to malfunction with variable severity. The condition is life threatening. Although most people with infection do not have and will not develop sepsis, non-specific signs and symptoms can lead to late recognition of people who might have sepsis. We would like clinicians to “think sepsis” and recognise symptoms and signs of potential organ failure when they assess someone with infection, in a similar way to thinking “Could this chest pain be cardiac in origin?”
This guidance provides a pragmatic approach for patients with infection who are assessed in the community, emergency departments, and hospitals by a wide range of general and specialist healthcare professionals. It includes guidance on assessment of risk factors followed by a detailed structured assessment of potential clinical signs and symptoms of concern.
Definitions of sepsis have been developed,2 but these offer limited explanation on how to confirm or rule out the diagnosis in general clinical settings or in the community.3 Current mechanisms to diagnose sepsis and guidelines for use largely apply to critical care settings such as intensive care.2 We recognised a need for better recognition of sepsis in non-intensive settings and for the diagnosis to be entertained sooner.
While sepsis is multifactorial and rarely presents in the same way, the Guideline Development Group considered that use of an easy, structured risk assessment may help clinicians identify those most severely ill who require immediate potentially lifesaving treatment. This guideline ensures that patients defined as having sepsis by recent definitions are, as a minimum, assessed as moderate-high risk.2 This guidance is also about appropriate de-escalation if sepsis is unlikely and broad spectrum antibiotics or hospital admission are not appropriate.
This article summarises recommendations from the National Institute for Health and Care Excellence (NICE) guideline for the recognition, diagnosis, and management of sepsis in children and adults.4 Recommendations and the clinical pathway are available via the NICE website, and the UK Sepsis Trust tools are being revised to align with this guidance.5 This article is accompanied by an infographic, which displays the NICE guideline as a decision making tool.
Recommendations
NICE recommendations are based on systematic reviews of best available evidence and explicit consideration of cost effectiveness. When minimal evidence is available, recommendations are based on the Guideline Development Group’s (GDG) experience and opinion of what constitutes good practice.
Think sepsis
People with sepsis may have non-specific, non-localised presentations—such as feeling very unwell—and may not necessarily have a high temperature. [Based on the experience and the opinion of the GDG]
Pay particular attention to concerns expressed by the patient and his or her family or carers, such as changes from usual behaviour. Take extra care when people cannot give a good history, such as people with English as a second language or with communication problems. [Based on the experience and opinion of the GDG]
Assess people with any suspected infection to identify:
The possible source of infection
Factors that increase risk of sepsis (see box 1)
Any signs that are indicators for clinical concern, such as new onset abnormalities of behaviour, circulation, or respiration.
[Based on the experience and opinion of the GDG]
If making a remote assessment, identify factors that increase risk of sepsis (see box 1) or indications of clinical concern (such as new onset abnormalities of behaviour, circulation, or respiration) when deciding whether to offer a face-to-face assessment and the urgency of the assessment. [Based on the experience and opinion of the GDG]
Use a structured set of observations to assess people in a face-to-face setting to stratify risk if there is clinical concern and sepsis is suspected. Consider using an early warning score to assess people with suspected sepsis in acute hospital settings. [Based on the experience and opinion of the GDG]
Suspect neutropenic sepsis in patients who are having anticancer treatment and become unwell, and refer them immediately for assessment in secondary or tertiary care (see NICE clinical guideline CG151 on neutropenic sepsis6). [Based on evidence underpinning the NICE clinical guideline on neutropenic sepsis (CG151)]
Box 1: Factors that increase the risk of sepsis
People in the following groups are at increased risk of developing sepsis
People <1 year old or >75 years old, or very frail people
People who have impaired immune systems because of illness or drugs:
Chemotherapy for cancer treatment
Impaired immune function (such as those with diabetes or sickle cell disease, or people who have had a splenectomy)
Long term treatment with corticosteroids
Treatment with immunosuppressant drugs for non-malignant disorders, such as rheumatoid arthritis
People who have had surgery, or other invasive procedures, in the past six weeks
People with any breach of skin integrity (such as cuts, burns, blisters, or skin infections)
Injecting drug misuse
People with indwelling lines or catheters.
Women who are pregnant or have given birth or had a termination of pregnancy or miscarriage in the past six weeks, in particular, women who
Have impaired immune systems because of illness or drugs
Have gestational diabetes or diabetes or other comorbidities
Needed invasive procedures (such as caesarean section, forceps delivery, removal of retained products of conception)
Had a prolonged rupture of membranes
Either have or have been in close contact with people with group A streptococcal infection (such as scarlet fever)
Have continued vaginal bleeding or an offensive vaginal discharge
Neonates, particularly if there has been:
Invasive group B streptococcal infection in a previous baby
Maternal group B streptococcal colonisation, bacteriuria, or infection in the current pregnancy
Prelabour rupture of membranes
Preterm birth after spontaneous labour (before 37 weeks’ gestation)
Suspected or confirmed rupture of membranes for >18 hours in a preterm birth
Maternal intrapartum fever >38°C, or confirmed or suspected chorioamnionitis
Parenteral antibiotic treatment given to the woman for confirmed or suspected invasive bacterial infection (such as septicaemia) at any time during labour or in the 24 hour periods before and after the birth (this does not refer to intrapartum antibiotic prophylaxis)
Suspected or confirmed infection in another baby in the case of a multiple pregnancy
Risk stratification
The GDG found evidence of low quality and used this as the starting point for their recommendations. If a person has infection and there is clinical concern that he or she might have sepsis, perform a structured assessment using the history and physical examination to grade risk of severe illness or death from sepsis (see infographic).
Changes in behaviour, in particular a new altered mental state, are a strong risk factor for mortality [Based on very low quality evidence from observational studies and the experience and opinion of the GDG]
Increased respiratory rate is associated with poor patient outcome and diagnosis of infection, particularly because pneumonia is a common cause of sepsis
Extreme values of blood pressure are a cause of clinical concern. However, blood pressure should be interpreted in the context of a person’s previous blood pressure if this is known [Based on very low quality evidence from observational studies and the experience and opinion of the GDG]
Tachycardia is a risk factor for serious infections and sepsis, and for admission to intensive care and mortality [Based on very low quality evidence from observational studies and the experience and opinion of the GDG]
Fever may be a risk factor for sepsis, although some studies showed that a high proportion of patients with sepsis did not have a temperature. In particular, elderly people, people receiving cancer treatment, and those who are severely unwell with sepsis are less likely to develop a raised temperature. [Based on very low quality evidence from observational studies and the experience and opinion of the GDG]
Very high temperature is unusual in children, and therefore it is often indicative of bacterial infection. [Based on evidence underpinning the NICE guideline on fever under 5s (CG160)7]
Mottled or ashen appearance; cyanosis of skin, lips, or tongue; and a non-blanching rash of skin are markers of high risk for severe illness or death. [Based on evidence underpinning the NICE guidelines on meningitis and meningococcal septicaemia (CG102)8 and fever in under 5s (CG160)7]
Early management of people with suspected sepsis
The management pathway depends on setting, patient’s age, and outcome of the structured assessment. Again, the evidence base was generally of low quality.
Setting
Outside hospital, people with a low risk of illness or death following structured assessment can be managed in a community setting and should not be referred to hospital. People with moderate to high risk may be managed outside acute hospital settings depending on clinical assessment. All those with high risk of illness or death from suspected sepsis should be referred to hospital.
In acute hospital settings, the recommendations are similar. For people at highest risk of illness or death from suspected sepsis, a senior doctor or nurse should review the patient immediately. They may make an alternative diagnosis and avoid the inappropriate use of broad spectrum antibiotics.
Management
Those classified as having a high risk of sepsis require antibiotics and fluids and potentially other supportive care. Treatment must be delivered in a timely fashion and may require specialist and critical care input. Use point of care lactate testing to guide fluid therapy and potential involvement of critical care.
If a definitive condition or infection cannot be identified, structured reassessment should be repeated. The management of suspected sepsis without any high or moderate to high risk criteria should be based on clinical judgment.
Source of infection
As part of the initial assessment, look for a source of infection, including sources that might need surgical drainage. Target testing according to history and examination—for example, urine analysis for those with suspected urinary tract sepsis. Imaging of the abdomen and pelvis should be considered if no likely source is identified after clinical examination and initial tests. [Based on knowledge of the epidemiology of causes, and the experience and opinion of the GDG]
Guidelines into practice
Do you “think sepsis” for everyone who presents with suspected infection?
Do you use a structured assessment for anyone with risk factors for sepsis and clinical abnormalities?
Among those assessed in hospital with suspected sepsis, has a senior doctor reviewed the patient immediately if highest risk factors are present?
Are those at high risk from sepsis offered intravenous antibiotics and fluid resuscitation within one hour of assessment?
How patients were involved in the creation of this article
Lay members joined the committee to form the recommendations summarised here. Patient organisations including the UK Sepsis Trust, MRSA Action UK, Group B Strep Support, Fiona Elizabeth Agnew Trust, and Meningitis Research Foundation were among the registered stakeholders that were consulted at both scoping and development stages.
Their involvement shaped the scope of the guideline and questions to be asked, and two lay members were full members of the committee. The UK Sepsis Trust has produced clinical tools to aid guideline implementation.
Further information on the guidance
Methods
The Guideline Development Group (GDG) comprised a consultant in paediatric immunology and infectious diseases (chair), two consultants in intensive care medicine (including one in paediatrics), two consultants in emergency medicine (including one in paediatrics), a chair in paediatric infection, a corporate matron in patient safety, a consultant in acute and critical care medicine, a general practitioner, an acute physician/senior clinical research lead, a paediatric development manager (paramedic), and two lay members. Co-opted experts included a consultant in obstetrics and a consultant in medical microbiology.
The guideline was developed using standard National Institute for Health and Care Excellence (NICE) guideline methodology (www.nice.org.uk/article/pmg20/chapter/1%20introduction%20and%20overview). The GDG developed clinical questions, collected and appraised clinical evidence, and evaluated the cost effectiveness of proposed interventions and management strategies through literature review and economic considerations where possible. Quality ratings of the evidence were based on GRADE methodology (www.gradeworkinggroup.org). These relate to the quality of the available evidence for assessed outcomes rather than the quality of the clinical study. Where standard methods could not be applied, a customised quality assessment was done.
Limited evidence exists for identification and early management of sepsis in primary care or the emergency department, and only some of the critical care evidence was relevant or interpretable in a meaningful way for non-critical care settings.
Future research
Can early warning scores, such as NEWS (national early warning scores for adults) and PEWS (paediatric early warning score), be used to improve the detection of sepsis in pre-hospital settings, and in emergency departments?
Is it possible to derive and validate a set of clinical decision rules or a predictive tool to rule out sepsis which can be applied to patients presenting to hospital with suspected sepsis?
What is the clinical and cost effectiveness of procalcitonin (PCT) point of care tests at initial triage for diagnosis of serious infection and the initiation of appropriate antibiotic therapy?
What are the incidence, presentation, and management of sepsis in the United Kingdom?
What is the association between the NICE sepsis guideline and patient care processes and outcomes over the next five years?
Footnotes
Contributors: All authors contributed to the conception and drafting of this article, and to revising it critically. They have all approved this version. SNF is guarantor.
Funding: The National Guideline Centre was commissioned and funded by the National Institute for Health and Care Excellence to write this summary.
Competing interests: We declare the following interests based on NICE's policy on conflicts of interests (available at: www.nice.org.uk/Media/Default/About/Who-we-are/Policies-and-procedures/code-of-practice-for-declaring-and-managing-conflicts-of-interest.pdf): SNF is a clinical trial investigator for commercial clinical trials and all NIHR portfolio commercial studies, which include paediatric vaccination trials, paediatric viral infection trials, a pilot phase II study of corticosteroids in paediatric sepsis and a trial on an antimicrobial agent in paediatric bone and joint infections. SNF is also a co-investigator on a commercially funded trial on pneumococcal molecular epidemiology. SNF attended a meeting arranged by Astellas, Cubist, and Actelion on Clostridium difficile infections in infants, a flu vaccine advisory board hosted by AstraZeneca, and an Infectious Diseases Research Network meeting on sepsis biomarkers for children. The authors’ full statements can be viewed at: www.nice.org.uk/guidance/ng51/evidence/appendices-ag-2551523294
