Article Text
Abstract
OBJECTIVE To determine the cardiovascular and coronary risk thresholds at which aspirin for primary prevention of coronary heart disease is safe and worthwhile.
DESIGN Meta-analysis of four randomised controlled trials of aspirin for primary prevention. The benefit and harm from aspirin treatment were examined to determine: (1) the cardiovascular and coronary risk threshold at which benefit in prevention of myocardial infarction exceeds harm from significant bleeding; and (2) the absolute benefit expressed as number needed to treat (NNT) for aspirin net of cerebral haemorrhage and other bleeding complications at different levels of coronary risk.
MAIN OUTCOME MEASURES Benefit from aspirin, expressed as reduction in cardiovascular events, myocardial infarctions, strokes, and total mortality; harm caused by aspirin in relation to significant bleeds and major haemorrhages.
RESULTS Aspirin for primary prevention significantly reduced all cardiovascular events by 15% (95% confidence interval (CI) 6% to 22%) and myocardial infarctions by 30% (95% CI 21% to 38%), and non-significantly reduced all deaths by 6% (95% CI −4% to 15%). Aspirin non-significantly increased strokes by 6% (95% CI −24% to 9%) and significantly increased bleeding complications by 69% (95% CI 38% to 107%). The risk of major bleeding balanced the reduction in cardiovascular events when cardiovascular event risk was 0.22%/year. The upper 95% CI for this estimate suggests that harm from aspirin is unlikely to outweigh benefit provided the cardiovascular event risk is 0.8%/year, equivalent to a coronary risk of 0.6%/year. At coronary event risk 1.5%/year, the five year NNT was 44 to prevent a myocardial infarction, and 77 to prevent a myocardial infarction net of any important bleeding complication. At coronary event risk 1%/year the NNT was 67 to prevent a myocardial infarction, and 182 to prevent a myocardial infarction net of important bleeding.
CONCLUSIONS Aspirin treatment for primary prevention is safe and worthwhile at coronary event risk ⩾ 1.5%/year; safe but of limited value at coronary risk 1%/year; and unsafe at coronary event risk 0.5%/year. Advice on aspirin for primary prevention requires formal accurate estimation of absolute coronary event risk.
- aspirin
- coronary heart disease
- primary prevention
- meta-analysis
Statistics from Altmetric.com
Aspirin reduces the risk of non-fatal myocardial infarction by 34%,1 and in the setting of secondary prevention reduces non-fatal strokes by 31%, cardiovascular events by 27%, and cardiovascular deaths by 18%.1 The relative risk reduction by aspirin appears constant,1-3 and absolute benefit is therefore determined by absolute coronary heart disease or cardiovascular risk.3 Aspirin causes cerebral2 and other haemorrhages,4-6 and this risk seems constant and independent of coronary heart disease risk.2 ,3 The balance between benefit and harm is therefore related to absolute coronary heart disease risk.3 When used for secondary prevention, the benefit from aspirin readily outweighs possible harm from major haemorrhage,1 ,3 ,7 but in primary prevention the balance between benefit and harm is not clear cut.1 ,3 ,7 Benefit will probably exceed harm in those at high risk,3 ,7 but a safe threshold of coronary heart disease risk for primary prevention with aspirin has not been defined.1 The question is important, because aspirin certainly can prevent non-fatal myocardial infarction when used for primary prevention.4-6Furthermore, 6–9% of healthy people take aspirin regularly.8 ,9 The aims of this analysis were to define the threshold of absolute coronary heart disease risk at which aspirin treatment is safe, and to quantitate benefit and harm from aspirin treatment at different levels of coronary heart disease risk.
Methods
TRIALS
Randomised controlled trials of aspirin for primary prevention were sought by Medline search from 1985 onwards, using the termscardiovascular disease andaspirin, and by scrutiny of previous meta-analyses and review articles. Additional prespecified criteria were that the studies had to report total cardiovascular events, myocardial infarction, stroke, bleeding complications, and all cause mortality as primary or secondary end points. We found four studies, one of unifactorial design10 and three of multifactorial design.4-6 In one factorial study, aspirin was used alone or with warfarin,5 and only the results for aspirin alone were included to avoid possible interaction between treatments. From the trial reports we obtained the number of subjects, person-years of follow up, and rates for end points. Specific steps were not taken to identify unpublished studies, but a rigorous search carried out for a meta-analysis published in 1994 failed to find any.1
END POINTS
Myocardial infarction included definite fatal and non-fatal myocardial infarction, but not possible myocardial infarction, silent infarction, or angina.Stroke included definite and probable cerebral infarction, cerebral haemorrhage, and stroke of uncertain cause, but not transient ischaemic attacks.Cardiovascular events included myocardial infarction and stroke (as defined above) plus cardiovascular deaths. In the UK doctors study,10 we excluded deaths classed as “other vascular and related causes” (rheumatic endocardial, hypertensive disease, pulmonary embolism, aortic aneurysm, and other vascular).
Bleeding complications were not classified or reported uniformly in the four trials.11 To calculate an overall odds ratio for bleeding complications we used the end point that provided the best estimate for each trial. These were: for the US physicians study,4 bleeds that required transfusion or operation or were fatal; for the UK doctors study,10 all fatal and non-fatal bleeds tabulated; and for the thrombosis prevention trial (TPT)5 and the hypertension optimal treatment (HOT) trial,6 all bleeds tabulatedexcluding minor bleeds. Note that cerebral haemorrhages were not included as bleeding complications but were classed as strokes, for the reason given below. These best estimates for each trial were used to calculate a combined odds ratio for the risk of bleeding complications with aspirin. A uniform measure of absolute risk of bleeding could not be derived using data from all four trials. Bleeding complications were therefore classed as major bleeds—defined as non-cerebral bleeds that caused death, transfusion, or operation—which were reported for two trials4 ,5; and all non-minor bleeds—defined as all non-cerebral bleeds not classed as minor—which were reported for two trials,5 ,6 both of which used aspirin at a dose of 75 mg daily. For the second analysis described below, examining net benefit from aspirin, the absolute risk of cerebral haemorrhage was taken from a recent meta-analysis of primary and secondary prevention trials that had used reliable diagnostic methods.2 The excess risk of cerebral haemorrhage was 0.12/100 persons over 37 months, or 0.039%/year. (This meta-analysis has been misinterpreted as showing a risk of cerebral haemorrhage of 0.12%/year,3 but our estimate is correct (J He, personal communication).)
STATISTICAL ANALYSES
For each trial absolute benefit or harm was calculated by subtracting event rates in the aspirin group from those in the control group. Odds ratios and 95% confidence intervals (CI) were calculated by log transformation, and the χ2 test was used to assess heterogeneity between trials at p < 0.1. Treatment effects across trials were estimated with weighting to calculate combined odds ratios and 95% CI, using a fixed effects model.12 Combined rates and odds ratios were used for all four trials, with two exceptions. The rates for major bleeds and all for non-minor bleeds were the weighted means from only two trials each, as described above.
Two analyses were performed, the first to define thesafety of aspirin for primary prevention, and the second to determine best estimates for benefit, net of bleeding complications, at different levels of coronary heart disease risk.
Safety of aspirin
In this analysis the measure of benefit was all cardiovascular events, which includes cerebral haemorrhages. The measure of harm was major bleeds, as defined above, which excludes cerebral haemorrhages. Cerebral haemorrhage was handled in this way because it was diagnosed incompletely in some trials. In this analysis cerebral haemorrhage caused by aspirin reduces benefit from treatment rather than increasing harm. The alternative analysis, to strip cerebral haemorrhages out from cardiovascular events and count them as major bleeds, is appropriate for trials that used reliable methods to distinguish between haemorrhagic and non-haemorrhagic strokes.2 The absolute benefit from aspirin (reduction of all cardiovascular events) and harm attributable to aspirin (excess of major bleeds) was examined, assuming that the model of Lubsen and Tijssen holds.13 The reduction of cardiovascular events by aspirin was calculated using the combined odds ratio for cardiovascular disease events and assuming a constant relative risk reduction and hence a linear proportionate relation between benefit and absolute cardiovascular disease risk.1 ,3 The risk of major bleeds with aspirin was assumed to be constant and independent of cardiovascular disease risk,2 ,3 and was calculated using the odds ratio for bleeding complications from all the trials and the absolute risk of major bleeds from two trials.4 ,5 The 95% confidence regions for benefit and harm over the range of cardiovascular disease event risks were estimated by the equation described by Armitage and Berry.12 The 95% CI for the level of cardiovascular disease event risk at which benefit equals harm was calculated using a joint probability of 0.95 for harm and benefit. This joint probability is related to the intersections between 78% CI around estimates of benefit and harm.12
Net benefit from aspirin related to coronary heart disease event risk
In this analysis reduction in myocardial infarction was used as the main measure of benefit because this end point accounted for all the benefit (see Results). Assuming relative risk reduction to be constant (as above) and using the combined odds ratio for all trials, the absolute reduction in myocardial infarction and the number needed to treat (NNT) for five years to prevent one myocardial infarct were calculated for coronary heart disease event risks of 0.5%, 1.0%, and 1.5% a year. The benefit and NNT for preventing myocardial infarction net of cerebral haemorrhage, of cerebral haemorrhage plus major bleed, and of cerebral haemorrhage plus all non-minor bleeds were calculated at these same coronary heart disease event risk levels. The risk of all bleeding complications was assumed constant, independent of coronary heart disease risk. Confidence intervals for estimates of NNT were calculated as described by Altman.14
Results
TRIALS OF ASPIRIN FOR PRIMARY PREVENTION
Four randomised controlled trials, the US physicians health study,4 UK doctors study,10 thrombosis prevention trial,5 and the HOT study6included 48 540 people, of whom 25 133 were treated with aspirin. Characteristics of the trial populations are summarised in table 1. Points of note are difference in aspirin dosage, from 75 to 500 mg daily; only one trial (HOT) included women; and all participants in HOT had hypertension which was well controlled.6
Baseline characteristics of study populations
BENEFIT FROM ASPIRIN
Table 2 shows analyses for myocardial infarction, stroke, all cardiovascular events, and all cause mortality. In control groups the coronary heart disease event risk varied from 0.36%/year (HOT) to 1.33%/year (thrombosis prevention trial), and cardiovascular event risk from 0.67%/year (US physicians) to 1.71%/year (thrombosis prevention trial). There was no heterogeneity in relative risk reduction between trials for all cardiovascular events, stroke, or all cause mortality. There was significant heterogeneity in relative risk reduction for myocardial infarction (p = 0.03), which was attributable to the results of the UK doctors study (table 2). The analysis was continued despite this (see Discussion). The odds ratios and confidence intervals showed significant reductions overall in all cardiovascular events (by 15%) and in myocardial infarction (by 30%), a non-significant reduction in all cause mortality (by 6%), and a non-significant increase in stroke incidence (by 6%).
Absolute risk, benefit, odds ratios (OR), and 95% confidence intervals (CI) for cardiovascular events, myocardial infarctions, strokes, and all cause mortality
BLEEDING COMPLICATIONS
Table 3 shows best estimates of risk of bleeding for all four trials. The absolute rate in control groups varied widely, from 0.05%/year to 0.46%/year, because of different definitions. The odds ratios varied from 1.05 to 1.90, with no significant heterogeneity, and the overall odds ratio was 1.69 (95% CI, 1.38 to 2.07). For major bleeds (two trials),4 ,5 the rates in control groups were identical (0.05%/year) and the odds ratio for aspirin treatment was 1.73 (95% CI 1.14 to 2.63). For all non-minor bleeds (two trials),5 ,6 the rates in control groups differed greatly (0.18%/year and 0.46%/year), and the odds ratio for aspirin was 1.77 (95% CI 1.40 to 2.25).
Absolute risk, harm, odds ratios (OR), and 95% confidence intervals (CI) for best overall estimate of haemorrhage, major bleeds, bleeds requiring transfusion, and non-minor bleeds
SAFETY OF ASPIRIN
Figure 1 shows the reduction in all cardiovascular events by aspirin, related to cardiovascular event risk, assuming a constant 15% relative risk reduction (table 2); the excess of major non-cerebral bleeds related to aspirin, assuming independence from cardiovascular risk; and the 95% confidence limits. By extrapolation, benefit equals harm at a cardiovascular event risk of 0.22%/year. The upper 95% confidence limit for the cardiovascular event threshold at which benefit equals harm was 0.8%/year; this cardiovascular event risk is equivalent to a coronary heart disease event risk of 0.6%/year.15 This analysis was repeated excluding the data from the UK doctors study, which was the source of significant heterogeneity. This made no important difference to the point at which benefit equalled harm for aspirin treatment, which was a cardiovascular event risk of 0.21%/year.
Absolute benefit (reduction in all cardiovascular events) (line A) and absolute harm (increase in major bleeds) (line B) from aspirin treatment, related to absolute cardiovascular event risk. The dotted lines show the 78% confidence regions. By extrapolation, benefit and harm from aspirin are equal when cardiovascular event risk is 0.22%/year, with an upper 95% confidence limit for this estimate at a cardiovascular event risk of approximately 0.8%/year.
NET BENEFIT AND NNT FOR ASPIRIN RELATED TO CORONARY HEART DISEASE EVENT RISK
Table 4 sets out benefit (number of myocardial infarctions prevented and NNT) net of bleeding complications of different severity, assuming that 100 people are treated for five years, for coronary heart disease event risks of 0.5%, 1.0%, and 1.5% a year. At a coronary heart disease event risk of 0.5%/year, the NNT to prevent a myocardial infarction is 133, and the NNT to prevent myocardial infarction without cerebral haemorrhage or a major bleed causing death, transfusion, or operation is 256. If all non-minor bleeding complications are considered, there is net harm from aspirin treatment at that coronary heart disease risk, with a number needed to harm (NNTH) of 500. At a coronary heart disease event risk of 1.5%/year the NNT to prevent a myocardial infarction is 44, and to prevent a myocardial infarction without cerebral or major haemorrhage, 53. If all non-minor bleeding complications are included there is still benefit, with a NNT net of any complication of 77 (69 to 88). At the intermediate coronary heart disease risk level, 1.0%/year, there is net benefit from aspirin treatment even if all non-minor bleeds are considered (table 4). However, benefit is relatively small, with the NNT to prevent myocardial infarction 67, and to prevent myocardial infarction without cerebral or major haemorrhage, 88.
Absolute reduction in myocardial infarctions by aspirin treatment of 100 persons for five years, and NNT, assuming relative risk reduction of 30%; and benefit net of bleeding complications of different severity
Discussion
There were difficulties in performing this meta-analysis. End points for benefit were reasonably uniform among the trials, but the analysis for myocardial infarction had to be forced through despite significant heterogeneity. This is a concern because prevention of myocardial infarction was entirely responsible for the significant reduction in all cardiovascular events. Significant heterogeneity could result from differences in study design, aspirin dose, type of people studied, compliance, or other factors (table 1), but the reason is not obvious. It may be a chance observation. Diagnosis of cerebral haemorrhage was incomplete in some trials—hence the decision to embed these complications within strokes rather than strip them out. This method of analysis is accurate numericallywhen defining the relation between benefit and harm, but may not be accurate quantitatively if cerebral haemorrhages are more severe than non-haemorrhagic strokes. There was some evidence for this in the UK doctors study, as strokes on aspirin were more often disabling than those in the control group.10 Reporting of bleeding complications in the four trials was extremely diverse.11 However, odds ratios for bleeding complications were similar whichever end point was used, and the overall odds ratio (1.69) was close to that for cerebral haemorrhage (1.84) reported in another meta-analysis.2
The estimate of relative risk for bleeds is probably reliable. There is much less certainty for the absolute risk of bleeding complications in the control groups. For each of the categories, major bleeds and all non-minor bleeds, only two trials could be used, and the absolute risk of all non-minor bleeds in control groups differed substantially, presumably because of differences in classification. The combined estimate for all non-minor bleeds must therefore be regarded with caution. It was retained because it provides the most conservative estimate of balance between benefit and harm, and also describes the outcome with aspirin 75 mg daily,5 ,6 the dose now widely used. Our conclusions rely on assumptions that the relative risk reduction with aspirin is constant, so that benefit is linearly related to absolute risk,1 ,3 whereas the absolute risk of bleeding is constant and independent of coronary or cardiovascular risk.2 ,3 These assumptions are valid as far as they have been tested,1-3 but the data are insufficient to test them rigorously.
A subgroup analysis of the thrombosis prevention trial published recently16 showed significant differences in benefit from aspirin according to systolic blood pressure, age, and serum cholesterol, findings that might cast doubt on the constancy of relative risk reduction by aspirin. However, these findings were not consistent in subgroup analyses of the US physicians study4 and the HOT study,17 and indeed in some instances are entirely inconsistent.18 There is a need to examine the relation of aspirin benefit to pretreatment coronary heart disease risk in greater depth, perhaps using the data for individual participants from all available trials.19Finally, those studied are not representative of the whole population. Relatively few women were included, and the findings apply only to hypertension that is well controlled.5 ,6
Given these difficulties why perform the analysis at all? These trials have shown substantial reductions in non-fatal myocardial infarction,4-6 ,19 and this has to be translated to ordinary practice. Advice that aspirin may be prescribed for primary prevention to those at high coronary risk3 ,7 is of little value unless “high coronary risk” is defined. Furthermore 6–9% of the population take aspirin regularly,8 ,9 and those with low coronary risk may come to serious harm. Given the difficulties discussed above, conclusions should err on the conservative side. We have tended to underestimate benefit by excluding end points such as prevention of transient ischaemic attacks or angina,10which are important. We have probably overweighted harm. We suspect that most people would prefer a myocardial infarct to a cerebral haemorrhage, but most would choose a non-cerebral haemorrhage needing transfusion over a myocardial infarct. By according major haemorrhages and major cardiovascular events equal weight in the analysis of safety we probably understate the value of preventing cardiovascular events. In the analysis of safety we used the upper 95% confidence limit to define the level of coronary heart disease risk at which it is reasonably certain that harm from aspirin will not exceed benefit.
Aspirin for primary prevention is more likely to do good than harm provided the cardiovascular event risk is ⩾ 0.8%/year, equivalent to a coronary heart disease event risk ⩾ 0.6%/year.15Table 4 shows that aspirin at a coronary heart disease event risk of 0.5%/year is unattractive. The five year NNT to prevent a myocardial infarction is high (133), and the NNT for benefit without a major haemorrhagic complication is 256. Numerically the risk of major plus all non-minor bleeding outweighs benefit. Aspirin treatment at this coronary heart disease risk level is not justified. At a coronary heart disease event risk of 1.5%/year (table 4) the outcome appears acceptable, with a five year NNT of 44 to prevent a myocardial infarct, and of 53 to prevent a myocardial infarct without a cerebral or major haemorrhage. Benefit exceeds harm even if all non-minor bleeds are included. At an intermediate coronary heart disease event risk level, 1%/year, benefit is relatively small (table 4). We suggest that people with coronary heart disease event risk of 1.5%/year or higher with no contraindication to aspirin should be identified for treatment. Individuals with low coronary heart disease risk, below 1.0%/year, should not be treated.
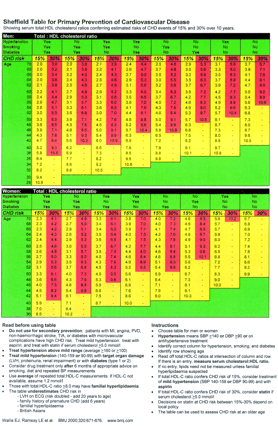
Aspirin cannot be prescribed safely for primary prevention of coronary heart disease without formal estimation of coronary disease event risk of the individual. Intuitive assessment of coronary heart disease risk20 and reliance on single risk factors such as lipids or blood pressure21 ,22 are highly inaccurate. Simple counting of coronary heart disease risk factors improves accuracy,22 but still identifies people at very low risk and fails to identify all high risk people for treatment.23 Accurate risk estimation requires counting and weighting of major risk factors for coronary heart disease,22 using risk functions derived from epidemiological studies such as Framingham.21 Aspirin treatment for primary prevention should be guided by formal estimation of coronary heart disease risk using the full Framingham equation,21 or simple methods based on Framingham.15 ,24 This is recommended in recent joint British societies' guidelines for coronary heart disease prevention24 and British Hypertension Society guidelines.25 In these guidelines the risk threshold for aspirin use for primary prevention is set at coronary heart disease event risk ⩾ 15% over 10 years, which is identical to the 1.5%/year coronary disease risk threshold identified in the present analysis. We have revised the Sheffield table15 to implement these guidelines, and to show the coronary heart disease risk threshold (15% over 10 years, equivalent to 1.5%/year) at which aspirin treatment is indicated (fig 2). This table has a sensitivity of 97% for detecting a coronary heart disease event risk of ⩾ 15% over 10 years, and will not identify for aspirin treatment any individual with a calculated coronary disease event risk below 10% over 10 years.15
Sheffield table for primary prevention of cardiovascular diseases