Article Text
Abstract
Objective Virtual chromoendoscopy (CE) is expected to enhance adenoma yield and reduce variation in performance between colonoscopists. This study aimed to compare the efficacy of narrow band imaging (NBI), flexible spectral imaging CE (FICE) and white light (WL) colonoscopy and their impact for less experienced endoscopists.
Methods We performed a randomised tandem colonoscopy trial controlling for withdrawal time and bowel preparation. Average-risk adults undergoing screening colonoscopy were enrolled and randomly assigned to first withdrawal with one of the three imaging modalities (NBI (NBI-WL group), FICE (FICE-WL group) and WL (WL-WL group)). Eight colonoscopists were categorised into expert and non-expert subgroups.
Results 1650 subjects (mean age 51.4 years, 63.9% men) were included (550 in each group). Compared with WL, neither NBI nor FICE increased the mean number of adenomas detected per patient (0.37 vs 0.35 and 0.36; p=0.591) or the percentage of patients with adenoma (25.3% vs 24.5% and 23.6%; p=0.753). For all three modalities, expert subgroups had higher yields of adenomas than non-expert subgroups. Learning curves were observed only for non-expert subgroups with all three modalities. The percentage of missed adenomas did not differ between the three groups (20.8% by WL vs 22.9% by NBI and 26.0% by FICE, p=0.300) and was not affected by endoscopists’ expertise.
Conclusions Neither NBI nor FICE improved adenoma detection or miss rates, with no difference in diagnostic efficacy between the two systems. Virtual CE had no additional benefits over WL for non-experts.
Clinical trial registration number: KCT0000570.
- Colonoscopy
- Screening
- Adenoma
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
-
Conventional colonoscopy reveals limitations in cancer prevention with an unacceptably high miss rate of adenomas.
-
Virtual chromoendoscopy (CE) such as narrow band imaging (NBI) and flexible spectral imaging CE (FICE) is expected to enhance adenoma yield and reduce variation in performance between colonoscopists.
-
Inconsistent results in the ability to detect colorectal adenomas could partially be explained by the preponderance of studies from high-risk populations performed only by experts at academic centres.
What are the new findings?
-
There were no differences in adenoma detection or miss rates between NBI and FICE systems.
-
For non-expert endoscopists, neither NBI nor FICE had any further benefit in diagnostic efficacy over WL.
How might it impact on clinical practice in the foreseeable future?
-
These findings do not corroborate the incorporation of virtual CE into routine clinical practice for screening of adenomas.
-
As yet, optimisation of operator performance might be of greater relevance and is a prerequisite before adoption of advanced technology.
Introduction
Emerging concerns about the incomplete degree of cancer prevention by conventional colonoscopy and its unacceptably high miss rate continue to provide an impetus for the development of new optical technologies.1–3 To optimise the potential of white light (WL) colonoscopy and to circumvent some drawbacks in conventional chromoendoscopy (CE), auxiliary virtual imaging techniques such as narrow band imaging (NBI) and flexible spectral imaging CE (FICE) have been proposed.4–6 In the NBI system the bandwidth of spectral transmittance is narrowed by optical filters, whereas the newer FICE endoscope processor estimates and modifies it arithmetically. Although NBI and FICE seem to achieve comparable imaging features, available research directly comparing these two systems is scarce. For predicting real-time histology of a colorectal polyp, virtual CE with or without magnification appears to be an effective substitute for conventional CE.7–11 On the other hand, less consistent results have been achieved for its ability to detect colorectal neoplasms.10 ,12–15 Recent large-scale randomised trials including our former study and systematic reviews failed to demonstrate the superiority of virtual CE over WL.16–23 This could partially be explained by the preponderance of selected papers from high-risk populations performed exclusively by experts at academic centres. Since the adenoma detection rate can function as a quality indicator for colonoscopy and a wide range of rates were reported by individual endoscopists, reducing the variation among examiners is a crucial issue.24 ,25 Few studies have formally investigated the impact of virtual CE on the operator. Whether colonoscopists with limited experience may benefit from virtual CE deserves additional evaluation in a non-specialised clinical setting. The aims of this study were (1) to compare the efficacy of NBI, FICE and WL in adenoma detection and miss rates during screening colonoscopy in an average-risk population and (2) to evaluate the diagnostic yields of virtual CE by the level of expertise while calculating the operators’ learning curves.
Methods
Study design and population
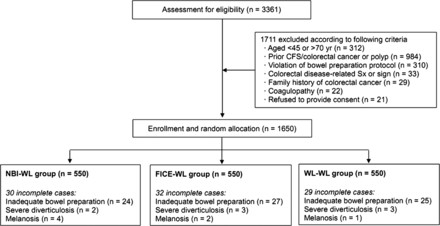
A prospective randomised controlled trial of tandem colonoscopy was performed at Seoul National University Hospital (SNUH) Healthcare System Gangnam Center. Figure 1 depicts the detailed enrollment protocol by randomisation arm. Asymptomatic healthy individuals aged 45–70 years who underwent screening colonoscopy were considered eligible for inclusion and were recruited consecutively between July 2011 and June 2012. All those being screened were requested to complete a structured questionnaire on gastrointestinal symptoms as well as medical histories before examination. Further information was ascertained by trained nurses regarding the reasons for colonoscopy and prior diagnosis of colorectal polyps. Based on the responses to the questionnaire, we excluded those who had colorectal disease-related symptoms or signs (eg, recent change in bowel habit, unexplained weight loss, anaemia, faecal occult blood test positivity or lower gastrointestinal tract bleeding not attributable to haemorrhoids), a family history of colorectal cancer (at least one first-degree relative with colorectal cancer diagnosed at any age) and past history of colorectal cancer or polyp or inflammatory bowel disease. Additional exclusion criteria were coagulopathy and significant violation of the bowel preparation protocol.
Study protocol and flow diagram of enrollment by randomisation arm according to the CONSORT (Consolidated Standards of Reporting Trials) statement. NBI, narrow band imaging; WL, white light; FICE, flexible spectral imaging chromoendoscopy.
Endoscopic equipment and endoscopists
We used a commercially available NBI system (CF-H260 series colonoscopes and CV-260SL video processor with CLV-260SL xenon light source; Evis Lucera Spectrum system: Olympus Optical Co, Tokyo, Japan).11 For NBI function, narrowed bands (30 nm wide spectra) of green (540 nm) and blue (415 nm) lights were used after optical filtering. The FICE system has been described previously and is equipped with EC-590ZW series colonoscopes, a VP-4400 video processor and XL-4400 light source (Fujinon, Saitama, Japan).16 FICE set 3 (R 540 nm, G 490 nm and B 420 nm) was exclusively chosen in our study based on prior data.16 All procedures including WL colonoscopy were performed using high-definition (HD) scopes without optical magnification. Of eight board-certified gastroenterologists (SJC, DK, MJP, YSK, JHS, HYK, JC and GEC), five were categorised as expert (mean of 8000 colonoscopies (range 6000–12 000) over a mean of 12.5 years (range 9–16 years)) and three were categorized as non-expert (more limited practice of about 1500 colonoscopies over <3 years). Pre-study training was carried out of at least five examinations with each of the virtual CE techniques in order to become acquainted with its colour characteristics in normal mucosa, stool or colonic pathology.
Colonoscopy procedures
All subjects underwent a 3-day dietary restriction and bowel preparation consisting of polyethylene glycol (PEG) lavage with 4 L during the 12 h preceding the procedure. Antispasmodic medication (scopolamine, 10 mg) was administered intramuscularly unless contraindicated. Those who chose conscious sedation were given midazolam intravenously—0.06 mg/kg at baseline and, according to sedation scale, additional 0.5–1.0 mg increments during the examination. Following enrollment and immediately prior to the examination, subjects were randomly allocated in a 1:1:1 ratio to one of the three study arms by opening a sealed opaque envelope containing a note with ‘NBI’, ‘FICE’ or ‘WL’. The colonoscopy was conducted in a modified back-to-back fashion by the same endoscopist. After complete insertion of the colonoscope into the caecum with WL and confirmation of sufficient bowel preparation, three colonic segments (ascending, transverse and descending to sigmoid colons) were sequentially inspected twice during withdrawal: (1) NBI-WL group: initially with NBI followed by re-examination of the same segment with WL; (2) FICE-WL group: the first examination was performed with FICE and the second examination with WL; and (3) WL-WL group: both examinations were performed by WL. The quality of the bowel preparation was rated at each segment as (a) excellent (clean and empty), (b) good (clear fluid), (c) fair (brown fluid but no residue after aspiration) and (d) poor (semisolid or solid stool).16
We attempted to adjust withdrawal times to 6 min (2 min for each segment) regardless of group assignment, with the exception of the time required for suctioning and irrigation, biopsy or polypectomy.26 The target area did not include rectum, which shows a high proportion of diminutive hyperplastic polyps. The clinical relevance of these lesions is limited in relation to the extent of time needed for biopsies. All lesions identified during the first or second withdrawal were numbered and photographed at equivalent angles and distances from the target mucosa. At the same time, the detailed polyp location was estimated according to the insertion distance of the colonoscope and anatomical landmarks. Immediately after completion of the second withdrawal, the endoscopist determined whether detected lesions were identical between the first and second withdrawals based on their images and locations. The following parameters were documented: examination time (both for instrument insertion and withdrawal), polyp characteristics such as number, location, size (measured by open-biopsy forceps) and macroscopic Paris classification.27 All detected polyps were removed endoscopically during the second withdrawal (diminutive polyps <5 mm by biopsy forceps and larger ones by endoscopic mucosal resection) and reviewed by two gastrointestinal pathologists who were unaware of the clinical information or endoscopic characteristics according to the WHO criteria.28
Statistical analysis
The primary end point of the study was to evaluate whether virtual CE could improve either adenoma detection (the number of adenomas per patient and the proportion of patients with at least one adenoma) or miss rate, and to compare the diagnostic yields between the NBI and FICE systems. Secondary outcome measures included the adenoma detection and miss rates and learning curves by the level of expertise. A detected lesion was defined as one observed during the first withdrawal with corresponding imaging modality. Assuming a 25% prevalence of adenoma-bearing patients with WL from our previous data and a 10% incremental difference from NBI or FICE application, 550 subjects per group would be required (80% power, significance level 0.05 and 10% dropout rate).29 Intention-to-treat analysis was performed using the full set of subjects including all incomplete cases that were deemed ineligible (ie, inadequate bowel preparation, severe diverticulosis and melanosis) after randomisation. A pooled miss rate was determined as the number of lesions identified during the second withdrawal only divided by the total number of lesions found in either the first or second withdrawal. In secondary analyses we compared the adenoma yields between the expert and non-expert subgroups in each imaging modality. To ascertain the influence of the experience of endoscopists with NBI and FICE on the detection of lesions, the learning curve was calculated by examining the changes in adenoma detection rates between the first and second halves of the study cases. The midway point was set up after the completion of enrollment by dividing the total number of cases of each endoscopist in half.
The data were stratified by the three imaging modality groups. Continuous variables were expressed as mean±SD and nominal and ordinal variables were described as proportions and percentages. The Wilcoxon rank sum test and χ2 test or Fisher exact test were used to compare the means and proportions as appropriate. For comparison among the three imaging modality groups, ANOVA or the Kruskal–Wallis test was applied for normally distributed and non-normally distributed continuous variables, respectively. To compare the miss rates while correcting for a lack of independence among multiple polyps identified in a person, logistic regression using Generalised Estimating Equations was performed. Statistical analysis was supported by the SNUH Medical Research Collaborating Center using statistical analysis package SAS V.9.2 (SAS Institute, Cary, North Carolina, USA). Differences with a two-tailed p value of <0.05 were considered statistically significant. Where there were three planned comparisons, p values were adjusted with a Bonferroni correction (p<0.017).
Results
Characteristics of study population
The flow diagram of enrollment is presented in figure 1 by randomisation arm. Among the 3361 subjects initially assessed for eligibility, 1711 did not meet the inclusion criteria. The remaining 1650 subjects were enrolled and were randomly assigned into one of the three imaging modality groups (550 in each group). Overall, the mean age of the study population was 51.4±8.4 years and 63.9% were men. The caecum was reached in 100% of the cases and no immediate or delayed procedure-related complications occurred. Ninety-one of the subjects did not complete the study protocol due to inadequate bowel preparation, severe diverticulosis and melanosis (30 in the NBI-WL group, 32 in the FICE-WL group and 29 in the WL-WL group). No significant differences were found between the three groups with regard to demographic features, the proportion of sedation colonoscopies, rating of bowel preparation and insertion time, as detailed in table 1.
Demographic and clinical characteristics of 1650 participants who underwent tandem colonoscopies for screening by randomisation arm
Adenoma detection rate
A total of 1223 colonic polyps were identified and retrieved for histological analysis from the entire study population, comprising 769 (62.9%) adenomas and 195 (15.9%) hyperplastic polyps. Five hundred and nine subjects (30.8%) had at least one adenoma during the first and second withdrawals. Overall, the adenoma detection rate with any of the three modalities was 24.5% (590 adenomas in 404 subjects) during the first withdrawal. For the primary outcome measures, there were no major differences between the three groups (NBI vs FICE, NBI vs WL or FICE vs WL); neither NBI nor FICE increased the mean number of adenomas per patient (0.35 and 0.36 vs 0.37; p=0.591) or the percentage of patients with at least one adenoma (24.5% and 23.6% vs 25.3%; p=0.753) compared with WL (table 2). Likewise, no significant differences were seen between the three imaging techniques with regard to the proportion of patients with multiple (≥3) adenomas and those with hyperplastic polyps (7.5% and 5.8% vs 6.9%; p=0.701).
Detection rates for overall polyps and adenomas at the first withdrawal by the type of imaging technique: per patient analysis
In secondary analyses, the expert subgroups consistently showed a larger number of adenomas and a higher percentage of patients with adenoma than the non-expert subgroups regardless of imaging technique. Also, the experts had a tendency to detect multiple adenomas, although not always significant (table 3). Over the two consecutive periods the adenoma detection rates for the expert subgroups remained unchanged for all three imaging modality groups. In contrast, in the non-expert subgroups a significantly higher proportion of adenomas were detected during the second period, which can be explained as a learning effect with all three imaging techniques by less experienced endoscopists (figure 2). In the expert subgroups, WL allowed a marginally (albeit non-significantly) higher yield of adenomas than NBI and FICE after post hoc analysis (0.58 vs 0.46 and 0.47, p=0.035 and 0.067; 36.5% vs 29.8% and 28.5%, p=0.023 and 0.034, respectively) (see online supplement 1). When sensitivity analysis was performed for 763 subjects with good to excellent bowel preparation, virtual CEs did not further improve the adenoma detection rates irrespective of the endoscopists’ level of experience, consistent with the primary finding (see online supplements 2 and 3).
Detection rates for overall polyps and adenomas in each randomisation arm by the level of expertise: per patient analysis
Learning curves for narrow band imaging (NBI), flexible spectral imaging chromoendoscopy (FICE) and white light (WL) by level of expertise. The adenoma detection rates for the expert subgroups remained stable throughout the course of the study period for all three imaging techniques whereas in the non-expert subgroup there was a significantly higher proportion of detected adenomas during the second half period with all three imaging techniques. *p<0.05 (first half vs second half).
Adenoma miss rate
The second inspection with WL yielded 288 additional polyps in 147 subjects and 179 adenomas in 84 subjects. The percentage of missed adenomas was 22.9% by NBI and 26.0% by FICE, and did not differ from the rate by WL (20.8%, p=0.300). Neither NBI nor FICE reduced the adenoma miss rate in the non-expert or expert subgroups (table 4). When analysing the endoscopic characteristics of missed lesions, there was no statistically significant difference by the type of imaging technique and by the level of expertise. The vast majority of missed polyps (n=273, 94.8%) were ≤5 mm in size and none had advanced histology (see online supplement 4). The mean size of overall missed adenomas was 3.4±1.6 mm, which was smaller than that of adenomas identified during the first withdrawal (4.4±2.4 mm, p=0.019) (data not shown).
Proportion of detected lesions at the first and second withdrawals in each randomisation arm by the level of expertise: per polyp analysis
Discussion
To our knowledge, this is the first randomised back-to-back trial to examine whether virtual CE outperforms conventional WL in detecting adenomas, directly comparing the two candidate imaging techniques (NBI vs FICE) by the level of expertise. Allowing for the exception of the rectum from the target area, our overall adenoma rate of 25% for WL is well within that of 25–40% from other centres.24 ,26 In this study virtual CE did not incrementally improve the detection or miss rates for adenomas over WL, without consideration of the experience of the endoscopists. Moreover, we did not find any substantial difference in efficacy between the NBI and FICE systems.
Although recent meta-analyses failed to prove an additional benefit of virtual CE for prespecified major outcomes, some controversy exists about combining the conflicting results from heterogeneous studies.18–23 The first trial from a US referral centre performed by a single expert reported a very high adenoma rate (around 60%) for HD-WL and this rate was not increased further by NBI.12 A similar result was shown in a German multicentre trial involving six experienced examiners but did not exhibit such a high rate, being more in line with our data.30 In the most recent controlled trial at two academic centres, NBI and HD-WL found more adenomas than standard definition (SD)-WL with no difference between the two modalities.17 Our study also found no difference between the systems used, but differs from the aforementioned trials in that we enrolled only average-risk persons without a previous history of polyps, thereby minimising any selection bias that might have affected the neoplasm detection rate. The influence of virtual CE was systematically evaluated in less experienced endoscopists and compared with that of experts. We have previously suspected that the high detection rate by experts using either virtual CE or WL led to difficulty in proving the benefit of virtual CE over WL.16 However, the hypothesis that virtual CE improves detection of adenomas by colonoscopists with limited experience and thus reduces the variations in diagnostic performance between examiners cannot be supported by these results. Some postulated a ‘training effect’ from virtual CE leading to better recognition by WL, even though they were not designed and powered for adenoma detection only for overall polyp detection.13 In this study, the operators’ experience guaranteed stable adenoma rates throughout the course of the study period and the learning curves were seen only for non-experts with any of the three imaging techniques.
Back-to-back miss rate studies using NBI or FICE have shown contradictory results.16 ,20 ,31–33 Until now, a few randomised controlled trials on virtual CE have reported positive results for adenoma detection not accompanied by a decreased miss rate or vice versa, or shown restricted location to the right colon: due to the small sample size focusing on missed adenomas or an uneven distribution of allocation, these rates might have rather low precision with insufficient statistical power to detect differences.14 ,31 ,33 Allowing for the small size of missed lesions (mean 3.4±1.6 mm) in our screening setting, the 20.8% miss rate for WL lies within the rates of 13% (95% CI 8.0% to 18%) for adenomas 5–10 mm and 26% (95% CI 27% to 35%) for adenomas 1–5 mm reported in a systematic review with tandem colonoscopies.1 This larger study reconfirmed that a substantial number of adenomas are missed irrespective of imaging technique (22.9% and 26.0% for NBI and FICE, respectively) across expert and less experienced colonoscopists, and suggested that virtual CE does not reduce the miss rate. As a back-to-back study, the increase in adenoma numbers during the second pass may be due to just looking again, and therefore similar miss rates might have been expected for the three modalities. Another explanation for our uniform miss rate across the imaging techniques is that polypectomies during the second examination took more time and thus enabled additional polyps to be found at the second withdrawal. Notably, most of the adenomas missed during the first examination were of small size and thought to be of little clinical importance. This might be a reflection of strict enrollment criteria—namely, average-risk asymptomatic individuals undergoing their first colonoscopy screening. The phenomenon that a given technique initially shows good or variable results but some accuracy is lost over time is common in medical imaging studies and may be due to limited case numbers, referral centre performance, highly motivated clinical researchers in an academic setting with case selection and enthusiasm to report good results.
Meanwhile, there are some technical aspects inherent to the virtual CE technique that may account for the disappointing yields. First, the brightness of the virtual image is still not sufficient to ensure a good overview in a large luminal diameter of colon even if these systems are used with HD technology.5 ,6 Of particular interest in the current study were the minor differences in favour of WL for experts, which indicates that WL colour is easier to assess for colonoscopists with experience than the somewhat more sophisticated virtual CE, but this remains speculative and warrants further investigation. Moreover, intestinal fluid such as bile appears reddish in colour, similar to blood, and obstructs the view to a greater extent than WL.5 ,6 Therefore, even minor failings in bowel preparation make comprehensive and meticulous detection difficult when using NBI or FICE. The fact that bowel preparation was adequate but not perfect in half of the study subjects may have contributed to the poorer than expected visualisation performance with virtual CE.34 In addition, the perceived lack of objective benefit with virtual CE may be attributable to the HD component and, thus, our results may overestimate the effect of WL.12 ,17 ,34 ,35 Since most newer endoscopy systems such as those used in this study generally introduce multiple new technologies and provide all image-enhanced methods simultaneously, it might be challenging to determine which individual component led to improved yields from a composite or synergistic effect of combining a number of smaller improvements. Lastly, the structures enhanced by the virtual imaging technique are best appreciated by detailed inspection of targeted areas that were deemed suspicious during conventional colonoscopy, preferably in a magnification mode.7 ,8 ,11 ,36 The local use of virtual CE in its present form would be desirable for our endoscopic armamentarium as we move forward rather than routine pan-colonic application.
The main strength of the study lies in the large sample size (over 1500 cases) resulting in stratification by important subgroups and well-powered evaluation of a relatively small number of missed adenomas. Another important advantage is the unique tandem design allowing for head-to-head comparison of the diagnostic yields between the two different virtual CE techniques (NBI vs FICE) accompanying WL as a reference standard. Furthermore, we tried to control the potential effect of both withdrawal time and bowel preparation on adenoma detection or miss rate.26 Limiting subjects to average-risk healthy individuals and several endoscopists with differing levels of experience make it possible to extrapolate our results to the real-world practice outside specialist units.
The interpretation of our findings requires careful consideration in several respects. First, the quality of bowel preparation in all three groups was not considered to be good to excellent in as many as 50%, which can lead to difficulty in showing the full effectiveness and diagnostic benefit of new optical devices. Sensitivity analysis for subgroups with good to excellent bowel preparation did not show any improvement in adenoma detection by virtual CEs, consistent with the primary analysis. In addition, we exclusively used the single-dose PEG regimen and subjects took their preparation during the 12 h preceding the procedure, which may partially explain a substantial proportion of the unsatisfactory bowel preparation.37 ,38 Future studies based on the consensus guidelines for bowel preparation are essential for understanding the value of the endoscopic imaging technique. Second, we selected modified back-to-back colonoscopy in a segmental manner and did not switch endoscopists between the first and second withdrawals. Future investigators might consider the use of a different endoscopy team, blinded to the results of the first examination, to perform the second examination. Third, WL imaging during insertion could contaminate the NBI and FICE data. However, such an effect represents an inherent potential bias in any study dealing with a comparison of new and conventional endoscopic technologies. Lastly, a single-centre design may reduce the generalisability and additional multicentre trials are warranted.
In summary, neither NBI nor FICE improved the adenoma detection or miss rates during screening colonoscopy and no difference was seen in diagnostic efficacy between the two systems. Virtual CE had no additional benefits over WL in any operator group, either experienced endoscopists or those less experienced. Accordingly, our findings do not corroborate the incorporation of virtual CE into routine clinical practice for screening of adenomas. We conclude that, at present, optimisation of operator performance might be of greater relevance and is a prerequisite before adoption of advanced technology.
Acknowledgments
We thank the SNUH Medical Research Collaborating Center for statistical advice.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online appendix
Footnotes
-
Contributors DK, SJC: study concept, design, analysis and interpretation of data. SJC, JHS, HYK, GEC, JC, DK, YSK and MJP: acquisition of data. SJC: drafting of the manuscript. SJC, DK, JHS, HYK, GEC, JC, YSK, MJP and JSK: critical revision of the manuscript.
-
Funding This work was supported by a grant from the Seoul National University Hospital Research Fund, grant number 04-2010-0440 (2010-1068).
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No H-1005-073-319).
-
Provenance and peer review Not commissioned; externally peer reviewed.