Article Text
Abstract
The authors report their initial clinical results with a novel wireless ocular telemetry sensor (OTS) (Sensimed AG, Switzerland) for continuous intraocular pressure (IOP) monitoring in patients with open angle glaucoma. This was a prospective, observational cohort of 15 patients. The OTS is a disposable silicone contact lens with an embedded micro-electromechanical system, which measures changes in corneal curvature induced by variations in IOP. An antenna, mounted around the eye, receives the data, which are then transmitted to a recorder. A signal was recorded in all patients. Thirteen (87%) patients completed 24-h IOP monitoring: one patient discontinued IOP monitoring due to device intolerance, and incomplete recordings were obtained in a second patient due to technical device malfunction. In 9/13 (69%) patients, the highest signals were recorded during the nocturnal period. No serious adverse events were recorded. The OTS shows good safety and functionality to monitor IOP fluctuations in patients over 24 h. This technology has the potential to provide hitherto unobtainable data on the chronobiology of IOP, possibly leading to improved care of glaucoma patients.
- Glaucoma
- deep sclerectomy
- goniopuncture
- in vivo confocal microscopy
- intraocular pressure
- continuous measurement
- contact lens tonometer
- diagnostic tests/investigation
Statistics from Altmetric.com
- Glaucoma
- deep sclerectomy
- goniopuncture
- in vivo confocal microscopy
- intraocular pressure
- continuous measurement
- contact lens tonometer
- diagnostic tests/investigation
Introduction
Intraocular pressure (IOP) is recognised as a major risk factor for the development of glaucoma and is at present the only modifiable risk factor. IOP varies throughout the diurnal and nocturnal periods and according to body posture.1 2 Liu et al were the first to demonstrate that in a majority of normal subjects and glaucoma patients the peak IOP occurred during the nocturnal period.2 3 Their findings were confirmed by numerous studies showing that IOP measurements during routine office hours fail to detect peak IOP in up to 62% of glaucoma patients.4 5 IOP fluctuation has been recognised as an independent risk factor for progression.6 However, even medically and surgically controlled patients show significant IOP fluctuations throughout the day and on provocative testing.7
Over 60 years after its invention by the Swiss ophthalmologist Goldmann, the eponymous applanation tonometer is still considered the gold standard for measuring IOP. However, its main limitation is impracticability to provide information on the 24-h IOP behaviour. In the past, attempts were made to find a practical solution for regular IOP monitoring, so far without success.8–12 Leonardi et al13 described a novel approach, which is based on the assumption that a correlation exists between IOP and corneal curvature. Studies have shown that an IOP variation of 1 mm Hg produces a change of central corneal curvature radius of approximately 3 μm.14 15 The key element of this measurement method is a soft contact lens with an embedded microfabricated strain gauge that allows the measurement of changes in corneal curvature. Previously, different studies have demonstrated good correlation between the recorded ocular telemetry sensor (OTS) signal and artificially induced IOP changes in enucleated porcine eyes and the ability to filter out noise in four human volunteers over 10 min monitoring (Pitchon et al IOVS 2008;48:ARVO E-Abstract 687).13 16 The commercial product (SENSIMED Triggerfish, Sensimed AG, Lausanne, Switzerland) obtained the CE mark in 2009. Herein, we describe our initial clinical experience with the OTS in glaucoma patients.
Methods
This was a prospective, uncontrolled, observational cohort of 15 consecutive patients at the Glaucoma Sector, University of Geneva. All included patients suffered from progressive open angle glaucoma (OAG) despite medical treatment and controlled IOPs during office hours. According to our clinical guidelines they were to undergo 24-h IOP monitoring. When offered the choice between monitoring under hospitalised conditions and ambulatory OTS monitoring, all preferred the latter. Informed consent was obtained. No approval from the institutional review board was required since the device is approved for clinical use and CE-marked as class IIa device.
The OTS is a disposable silicone contact lens with an embedded micro-electromechanical system and a thin microfabricated platinum–titanium strain gauge. Total thickness of the strain gauge is 7 μm. The sensing resistive gauges in the device have a circular arc shape around the centre, placed over a circumference of 11.5 mm diameter, which is the average of the corneoscleral junction position, where changes in IOP are assumed to induce maximum corneal deformation.13 Measurements are taken every 600 s for a duration of 60 s, giving a total of 144 measurements over a 24-h period. The results obtained are presented in an arbitrary unit and not mm Hg. The thickness of the sensor is less than 600 μm in the centre and 250 μm in the periphery. Currently, three base curves are available (flat 9.0, medium 8.7 and steep 8.4). The OTS is powered by radiofrequency waves at 27 MHz from the external antenna, which is embedded in the patch applied around the patient's eye. On the other hand, the OTS sends back to the external antenna the monitoring data that are then transmitted by wire to a recorder worn around the waist. The device is described in more detail elsewhere13 (figure 1). Patients were asked to grade their average level of ocular comfort on a 10-grade scale (0 signifying intolerable pain and 10 signifying perfect comfort) and to record their activities during monitoring in a logbook.
The wireless sensor is in place. A soft patch containing the antenna is applied around the eye and transmits the information via wire to the recorder that the patient wears in a pocket fixed around the neck and waist. The patient can continue to wear her spectacles during monitoring (patient consent was obtained for publication of this photograph).
Results
A signal was recorded in all 15 patients. Mean age was 63.7±13.5 (SD), 67% were male. Twelve patients suffered from primary OAG and three from pseudoexfoliative glaucoma. Thirteen (87%) patients finished complete 24-h IOP monitoring: one patient with pre-existent severe dry eye disease (DED) discontinued IOP monitoring after 13 h due to device intolerance, and monitoring was interrupted after 17 h in a second patient due to technical device malfunction. In 9/13 (69%) patients, the highest signals were recorded during the nocturnal period. Prolonged peaks (>1 h) were observed in 12/15 (80%) patients with 75% exclusively occurring outside office hours. No serious adverse events were recorded. One case of corneal erosion (1×1 mm) in a patient with severe DED and four cases of superficial punctate keratitis constituted minor complications. All resolved after 24 h. Average patient score for comfort was 7. Following the findings of the 24-h monitoring, therapy was changed in 11 patients (73%). Figures 2–5 present four typical curves.
The figure shows the computer interface of the SENSIMED Triggerfish signal when downloaded to the physician's computer at the end of the 24-h IOP monitoring. In the centre, the IOP curve over 24 h is shown. Each point on the graph represents 60 s of IOP measurement, repeated every 600 s. The user can place one of two cursors on any point in time and see the detailed measurements in the smaller windows at the bottom (each corresponding to a period of 60 s), where spikes due to blinking as well as ocular pulsations are clearly visible. A characteristic short-term signal drop is occasionally noticed and correlates well with exposure to sunlight during outdoor activities. The effect of blinking is visible in the detailed profile as a fraction-of-a-second peak (Zoom A). Ocular pulsations could be seen in all curves. In Zoom B, showing the registration signal during 60 s at night, it can be seen that the ocular pulsation frequency is in line with that of cardiac activity. The patient is asleep and no blinkings are seen. The patient instils his topical prostaglandin drops (PG) at around 22:00.
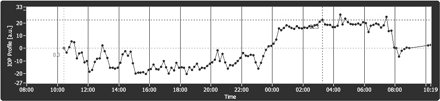
The graph shows the 24-h IOP curve of a 60-year-old male patient, suffering from primary open angle glaucoma (POAG). Goldmann applanation tonometry (GAT) measured an IOP of 13 mm Hg at baseline and 15 mm Hg at the end of 24-h SENSIMED Triggerfish monitoring. The curve shows a stable IOP during most of the day and evening. However, from 02:00 to 03:00 a significant and prolonged peak is registered, followed by a plateau and a second peak at 05:00–06:00. PG drops were instilled shortly after 22:00.
The graph shows the 24-h IOP curve of a 79-year-old female POAG patient under treatment with a PG, with GAT IOPs of 14 mm Hg (baseline) and 16 mm Hg (end of 24-h SENSIMED Triggerfish monitoring). The curve reveals a stable profile during the afternoon hours with a trough at 17:30. The signal then increases continuously during evening hours with significant fluctuations throughout the nighttime. A peak is observed at 01:00. This patient was under a topical treatment with twice-daily α-2-adrenergic agonist (instilled around 17:00 and 09:30, following day) and once-daily PG drops (instillation time not recorded by patient).
The graph shows the 24-h IOP curve of a 56-year-old male pseudoexfoliation glaucoma patient under a topical PG analogue. GAT IOPs were 21 mm Hg (baseline) and 23 mm Hg (end of 24-h SENSIMED Triggerfish monitoring). Important fluctuations are seen throughout the day, decreasing in amplitude during the afternoon. A sharp increase is observed at 23:00, followed by a plateau lasting from 00:30 to 07:30 with a peak at 04:30. According to the patient logbook, he applied his PG drops at 23:00 and went to bed at 23:30. This surprising increase immediately following PG application was observed in a second patient using the same PG drops. No such increase was observed after application of other drops. A sharp decrease is observed at 07:30–08:00 and corresponds to the patient's awakening and standing up.
Conclusion
At present, glaucoma management is mostly based on single IOP measurements obtained during routine office visits despite the fact that controlled studies performed in a sleep laboratory indicate that the habitual IOPs of most glaucoma patients are higher during the nocturnal/sleep period than during office hours.1–3 The reason for this is presumed to be the increase of episcleral venous pressure and the redistribution of body fluid.17 These findings, however, could be biased by the fact that measuring IOP itself during sleep is influenced by the effect of awakening.18 19 Moreover, static IOP measurements obtained with diurnal and nocturnal testing only estimate a portion of the 24-h IOP and do not reflect the dynamic nature of IOP.
Our initial clinical experience with the Triggerfish device has yielded a good safety and tolerability profile. The observed corneal changes compared well with published literature on contact lens complications.20 21 The data obtained were highly relevant and led to an immediate treatment change in two-thirds of patients. We believe that this OTS has the potential to improve clinical care of glaucoma patients in the same way that continuous blood pressure monitoring or home measurements of blood glucose levels have done for patients with high blood pressure or diabetes. Important questions need to be answered such as the effect of nighttime changes in corneal thickness and ocular movements on the precision of the device.
References
Linked Articles
- Editorial
- At a glance