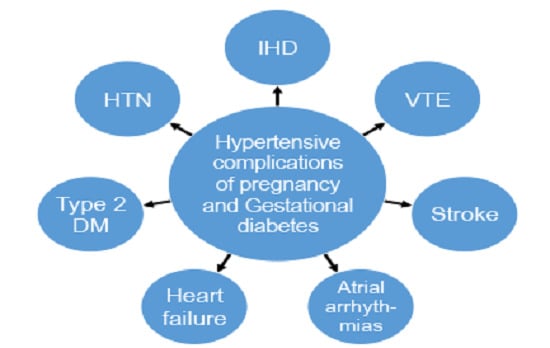
Cardiovascular Complications of Pregnancy
Abstract
:1. Introduction
2. Hemodynamic Changes during Normal Pregnancy
3. Metabolic Changes during Normal Pregnancy
3.1. Glucose Metabolism
3.2. Lipid Metabolism
4. Hypertensive Disorders of Pregnancy: Gestational Hypertension and Preeclampsia/Eclampsia
4.1. Diagnosis
4.2. Pathophysiology of Preeclampsia
4.3. Short Term Maternal and Fetal Effects of Preeclampsia and Eclampsia
4.4. Long Term Maternal Cardiovascular Risks Associated with Preeclampsia
5. Preterm Birth and Small for Gestational Age—Maternal and Offspring Consequences
6. Biomarkers Related to Hypertensive Disorders of Pregnancy
7. Gestational Diabetes
8. Predictive Models and Risk Reduction
9. Approaches to Intervention
10. Conclusions
Author Contributions
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the american heart association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Women’s Heart Disease Awareness Study. Available online: https://www.goredforwomen.org/about-heart-disease/facts_about_heart_disease_in_women-sub-category/womens-heart-disease-awareness-study-2012/ (accessed on 17 September 2015).
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Danilack, V.A.; Elston, B.; Lipkind, H.S. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am. J. Epidemiol. 2014, 180, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Pudwell, J.; Roddy, M.; Cho, C.K.; Smith, G.N. The maternal health clinic: An initiative for cardiovascular risk identification in women with pregnancy-related complications. Am. J. Obstet. Gynecol. 2014, 210, e431–e439. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.A. Changes in the blood volume during pregnancy and delivery. Anesthesiology 1965, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, A.C.; Stigter, R.H.; Bruinse, H.W. Cardiac output in normal pregnancy: A critical review. Obstet. Gynecol. 1996, 87, 310–318. [Google Scholar] [CrossRef]
- Curran-Everett, D.; Morris, K.G., Jr.; Moore, L.G. Regional circulatory contributions to increased systemic vascular conductance of pregnancy. Am. J. Physiol. 1991, 261, H1842–H1847. [Google Scholar] [PubMed]
- Geva, T.; Mauer, M.B.; Striker, L.; Kirshon, B.; Pivarnik, J.M. Effects of physiologic load of pregnancy on left ventricular contractility and remodeling. Am. Heart J. 1997, 133, 53–59. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Innes, B.A.; Levey, J.; Robson, S.C.; Lash, G.E. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012, 26, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mata, K.M.; Mazzuca, M.Q.; Khalil, R.A. Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sflt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem. Pharmacol. 2014, 89, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Easterling, T.R.; Benedetti, T.J.; Schmucker, B.C.; Carlson, K.; Millard, S.P. Maternal hemodynamics and aortic diameter in normal and hypertensive pregnancies. Obstet. Gynecol. 1991, 78, 1073–1077. [Google Scholar] [PubMed]
- Talbert, L.M.; Langdell, R.D. Normal values of certain factors in the blood clotting mechanism in pregnancy. Am. J. Obstet. Gynecol. 1964, 90, 44–50. [Google Scholar] [PubMed]
- Okoroh, E.M.; Azonobi, I.C.; Grosse, S.D.; Grant, A.M.; Atrash, H.K.; James, A.H. Prevention of venous thromboembolism in pregnancy: A review of guidelines, 2000–2011. J. Women’s Health 2012, 21, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Coppens, M.; Cattaneo, M.; Middeldorp, S. Pregnancy-related venous thromboembolism: Risk and the effect of thromboprophylaxis. Thromb. Res. 2012, 129, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Brennand, J.; Conkie, J.A.; McCall, F.; Greer, I.A.; Walker, I.D. Activated protein c sensitivity, protein c, protein s and coagulation in normal pregnancy. Thromb. Haemost. 1998, 79, 1166–1170. [Google Scholar] [PubMed]
- Brizzi, P.; Tonolo, G.; Esposito, F.; Puddu, L.; Dessole, S.; Maioli, M.; Milia, S. Lipoprotein metabolism during normal pregnancy. Am. J. Obstet. Gynecol. 1999, 181, 430–434. [Google Scholar] [CrossRef]
- Charlton, F.; Tooher, J.; Rye, K.A.; Hennessy, A. Cardiovascular risk, lipids and pregnancy: Preeclampsia and the risk of later life cardiovascular disease. Heart Lung Circ. 2014, 23, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Emet, T.; Ustuner, I.; Guven, S.G.; Balik, G.; Ural, U.M.; Tekin, Y.B.; Senturk, S.; Sahin, F.K.; Avsar, A.F. Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch. Gynecol. Obstet. 2013, 288, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.; Bertolotto, A.; Resi, V.; Volpe, L.; di Cianni, G. Triglyceride metabolism in pregnancy. Adv. Clin. Chem. 2011, 55, 133–153. [Google Scholar] [PubMed]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatal. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: A review. J. Am. Coll. Cardiol. 2014, 63, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Brichant, J.F.; Hartstein, G.; Bonhomme, V.; Dewandre, P.Y. Preeclampsia: An update. Acta Anaesthesiol. Belg. 2014, 65, 137–149. [Google Scholar] [PubMed]
- Vaughan, J.E.; Walsh, S.W. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens. Pregnancy 2002, 21, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Gant, N.F.; Chand, S.; Whalley, P.J.; MacDonald, P.C. The nature of pressor responsiveness to angiotensin II in human pregnancy. Obstet. Gynecol. 1974, 43, 854–860. [Google Scholar] [PubMed]
- Romero, R.; Mazor, M.; Lockwood, C.J.; Emamian, M.; Belanger, K.P.; Hobbins, J.C.; Duffy, T. Clinical significance, prevalence, and natural history of thrombocytopenia in pregnancy-induced hypertension. Am. J. Perinatal. 1989, 6, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.C.; Fraser, W.D. Longitudinal vitamin d status in pregnancy and the risk of pre-eclampsia. BJOG 2012, 119, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; McAllister, M.J.; Huang, C.J. A therapeutic role for vitamin d on obesity-associated inflammation and weight-loss intervention. Inflamm. Res. 2015, 64, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Tran, B.; Ebeling, P.R.; English, D.R.; Lucas, R.M.; Venn, A.J.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Effect of vitamin d supplementation on selected inflammatory biomarkers in older adults: A secondary analysis of data from a randomised, placebo-controlled trial. Br. J. Nutr. 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef]
- Maynard, S.; Epstein, F.H.; Karumanchi, S.A. Preeclampsia and angiogenic imbalance. Ann. Rev. Med. 2008, 59, 61–78. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Vitamin D: Screening and supplementation during pregnancy. Obstet. Gynecol. 2011, 118, 297–298. [Google Scholar]
- MacKay, A.P.; Berg, C.J.; Atrash, H.K. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet. Gynecol. 2001, 97, 533–538. [Google Scholar] [CrossRef]
- Roberts, C.L.; Algert, C.S.; Morris, J.M.; Ford, J.B.; Henderson-Smart, D.J. Hypertensive disorders in pregnancy: A population-based study. Med. J. Aust. 2005, 182, 332–335. [Google Scholar] [PubMed]
- Broekhuijsen, K.; Ravelli, A.C.; Langenveld, J.; van Pampus, M.G.; van Den Berg, P.P.; Mol, B.W.; Franssen, M.T. Maternal and neonatal outcomes of pregnancy in women with chronic hypertension: A retrospective analysis of a national register. Acta Obstet. Gynecol. Scand. 2015. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I.R.; Silva, W.B.; Cerqueira, G.S.; Novo, N.F.; Almeida, F.A.; Novo, J.L. Maternal and fetal outcome in women with hypertensive disorders of pregnancy: The impact of prenatal care. Ther. Adv. Cardiovasc. Dis. 2015, 9, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.M.; Mahfouz, E.M.; Gomaa, G.F.; El-Diasty, T.A.; Alldawi, L.; Ikrar, T.; Limin, D.; Kodama, M.; Aizawa, Y. Evaluation of coronary calcium score by multidetector computed tomography in relation to endothelial function and inflammatory markers in asymptomatic individuals. Circ. J. 2008, 72, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Flores, F.; Tsai, J.; Frandsen, T.; Yamamoto, H.; Takasu, J. Measures of brachial artery distensibility in relation to coronary calcification. Am. J. Hypertens. 2003, 16, 350–355. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Bielak, L.F.; Rule, A.D.; Sheedy, P.F.; Turner, S.T.; Garovic, V.D.; Peyser, P.A. Hypertension during pregnancy is associated with coronary artery calcium independent of renal function. J. Womens Health 2009, 18, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, E.B.; Vatten, L.J.; Smith, G.D.; Romundstad, P.R. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet. Gynecol. 2009, 114, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Garovic, V.D.; Bailey, K.R.; Boerwinkle, E.; Hunt, S.C.; Weder, A.B.; Curb, D.; Mosley, T.H., Jr.; Wiste, H.J.; Turner, S.T. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J. Hypertens. 2010, 28, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Lykke, J.A.; Langhoff-Roos, J.; Sibai, B.M.; Funai, E.F.; Triche, E.W.; Paidas, M.J. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009, 53, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Irgens, H.U.; Reisaeter, L.; Irgens, L.M.; Lie, R.T. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001, 323, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, L.S.; Arngrimsson, R.; Geirsson, R.T.; Sigvaldason, H.; Sigfusson, N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet. Gynecol. Scand. 1995, 74, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Shopen, N.; Schiff, E.; Koren-Morag, N.; Grossman, E. Factors that predict the development of hypertension in women with pregnancy-induced hypertension. Am. J. Hypertens. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Van Walraven, C.; Mamdani, M.; Cohn, A.; Katib, Y.; Walker, M.; Rodger, M.A. Risk of subsequent thromboembolism for patients with pre-eclampsia. BMJ 2003, 326, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Rodie, V.A.; Freeman, D.J.; Sattar, N.; Greer, I.A. Pre-eclampsia and cardiovascular disease: Metabolic syndrome of pregnancy? Atherosclerosis 2004, 175, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Gorostidi, M.; Portal, C.G.; Sanchez, M.; Sanchez, E.; Alvarez, J. Long-term prognosis of hypertension in pregnancy. Hypertens. Pregnancy 2000, 19, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Hermes, W.; Franx, A.; van Pampus, M.G.; Bloemenkamp, K.W.; Bots, M.L.; van der Post, J.A.; Porath, M.; Ponjee, G.A.; Tamsma, J.T.; Mol, B.W.; et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: A cohort study. Am. J. Obstet. Gynecol. 2013, 208, e471–e478. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasiry, S.; Ghossein-Doha, C.; Polman, S.; Lemmens, S.; Scholten, R.; Heidema, W.; Spaan, J.; Spaanderman, M. Metabolic syndrome after pregnancies complicated by pre-eclampsia or small for gestational age: A retrospective cohort. BJOG Int. J. Obstet. Gynaecol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kessous, R.; Shoham-Vardi, I.; Pariente, G.; Sergienko, R.; Sheiner, E. Long-term maternal atherosclerotic morbidity in women with pre-eclampsia. Heart 2015, 101, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Hermes, W.; Tamsma, J.T.; Grootendorst, D.C.; Franx, A.; van der Post, J.; van Pampus, M.G.; Bloemenkamp, K.W.; Porath, M.; Mol, B.W.; de Groot, C.J. Cardiovascular risk estimation in women with a history of hypertensive pregnancy disorders at term: A longitudinal follow-up study. BMC Pregnancy Childbirth 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Funai, E.F.; Friedlander, Y.; Paltiel, O.; Tiram, E.; Xue, X.; Deutsch, L.; Harlap, S. Long-term mortality after preeclampsia. Epidemiology 2005, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Redelmeier, D.A. Cardiovascular health after maternal placental syndromes (champs): Population-based retrospective cohort study. Lancet 2005, 366, 1797–1803. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, S.H.; Ho, C.H.; Liang, F.W.; Chu, C.C.; Wang, H.Y.; Lu, Y.H. End-stage renal disease after hypertensive disorders in pregnancy. Am. J. Obstet. Gynecol. 2014, 210, 147.e1–147.e8. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.M.; Cohn, B.A. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the child health and development studies pregnancy cohort. Circulation 2015, 132, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Aykas, F.; Solak, Y.; Erden, A.; Bulut, K.; Dogan, S.; Sarli, B.; Acmaz, G.; Afsar, B.; Siriopol, D.; Covic, A.; et al. Persistence of cardiovascular risk factors in women with previous preeclampsia: A long-term follow-up study. J. Investig. Med. 2015, 63, 641–645. [Google Scholar] [PubMed]
- Henriques, A.C.; Carvalho, F.H.; Feitosa, H.N.; Macena, R.H.; Mota, R.M.; Alencar, J.C. Endothelial dysfunction after pregnancy-induced hypertension. Int. J. Gynaecol. Obstet. 2014, 124, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Schull, M.J.; Kingdom, J.C.; Vermeulen, M.J. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart 2012, 98, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith, G.; Hypponen, E.; Power, C.; Lawlor, D.A. Offspring birth weight and parental mortality: Prospective observational study and meta-analysis. Am. J. Epidemiol. 2007, 166, 160–169. [Google Scholar] [CrossRef]
- Smith, G.D.; Harding, S.; Rosato, M. Relation between infants’ birth weight and mothers’ mortality: Prospective observational study. BMJ 2000, 320, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Whitley, E.; Gissler, M.; Hemminki, E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet 2000, 356, 2066–2067. [Google Scholar] [CrossRef]
- Catov, J.M.; Wu, C.S.; Olsen, J.; Sutton-Tyrrell, K.; Li, J.; Nohr, E.A. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann. Epidemiol. 2010, 20, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kessous, R.; Shoham-Vardi, I.; Pariente, G.; Holcberg, G.; Sheiner, E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am. J. Obstet. Gynecol. 2013, 209, e361–e368. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Pell, J.P.; Walsh, D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet 2001, 357, 2002–2006. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hart, C.; Ferrell, C.; Upton, M.; Hole, D.; Hawthorne, V.; Watt, G. Birth weight of offspring and mortality in the renfrew and paisley study: Prospective observational study. BMJ 1997, 315, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Blondon, M.; Quon, B.S.; Harrington, L.B.; Bounameaux, H.; Smith, N.L. Association between newborn birth weight and the risk of postpartum maternal venous thromboembolism: A population-based case-control study. Circulation 2015, 131, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Pariente, G.; Sheiner, E.; Kessous, R.; Michael, S.; Shoham-Vardi, I. Association between delivery of a small-for-gestational-age neonate and long-term maternal cardiovascular morbidity. Int. J. Gynaecol. Obstet. 2013, 123, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Stuart, J.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Stuebe, A.; Oken, E. Preterm birth and long-term maternal cardiovascular health. Ann. Epidemiol. 2015, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Peeters, L.L.; Stehouwer, C.D. Preeclampsia and increased blood pressure in the offspring: Meta-analysis and critical review of the evidence. J. Hypertens. 2009, 27, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P.J. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Heart J. 2008, 156, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Oglaend, B.; Forman, M.R.; Romundstad, P.R.; Nilsen, S.T.; Vatten, L.J. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J. Hypertens. 2009, 27, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Garcia, G.; Contag, S. Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr. Hypertens. Rep. 2014, 16, 475. [Google Scholar] [CrossRef] [PubMed]
- Palmsten, K.; Buka, S.L.; Michels, K.B. Maternal pregnancy-related hypertension and risk for hypertension in offspring later in life. Obstet. Gynecol. 2010, 116, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Kaijser, M.; Bonamy, A.K.; Akre, O.; Cnattingius, S.; Granath, F.; Norman, M.; Ekbom, A. Perinatal risk factors for ischemic heart disease: Disentangling the roles of birth weight and preterm birth. Circulation 2008, 117, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.C.; Black, M.A.; Poston, L.; Taylor, R.; Myers, J.E.; Baker, P.N.; McCowan, L.M.; Simpson, N.A.; Dekker, G.A.; Roberts, C.T.; et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: The screening for pregnancy endpoints (scope) international cohort study. Hypertension 2014, 64, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.C.; Broadhurst, D.I.; Dunn, W.; Brown, M.; North, R.A.; McCowan, L.; Roberts, C.; Cooper, G.J.; Kell, D.B.; Baker, P.N.; et al. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension 2010, 56, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Pare, E.; Parry, S.; McElrath, T.F.; Pucci, D.; Newton, A.; Lim, K.H. Clinical risk factors for preeclampsia in the 21st century. Obstet. Gynecol. 2014, 124, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Cowans, N.J.; Chefetz, I.; Tal, J.; Meiri, H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery doppler pulsatility index as markers of pre-eclampsia. Int. J. Gynaecol. Obstet. 2007, 29, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Thilaganathan, B.; Ralph, E.; Papageorghiou, A.T.; Melchiorre, K.; Sheldon, J. Raised maternal serum cystatin c: An early pregnancy marker for preeclampsia. Reprod. Sci. 2009, 16, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Casagrandi, D.; Livanos, P.; Tetteh, A.; Nicolaides, K.H. Maternal plasma pentraxin 3 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat. Diagn. 2009, 29, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, A.K.; Larsson, A.; Eriksson, U.J.; Nash, P.; Norden-Lindeberg, S.; Olovsson, M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet. Gynecol. 2007, 109, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Holthe, M.R.; Staff, A.C.; Berge, L.N.; Lyberg, T. Different levels of platelet activation in preeclamptic, normotensive pregnant, and nonpregnant women. Am. J. Obstet. Gynecol. 2004, 190, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Banzola, I.; Farina, A.; Concu, M.; Sekizawa, A.; Purwosunu, Y.; Strada, I.; Arcelli, D.; Simonazzi, G.; Caramelli, E.; Rizzo, N. Performance of a panel of maternal serum markers in predicting preeclampsia at 11–15 weeks’ gestation. Prenat. Diagn. 2007, 27, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, R.; Baviera, G.; Giordano, D.; Todarello, G.; Russo, S.; Recupero, S.; Bolignano, D.; Corrado, F. Neutrophil gelatinase-associated lipocalin serum evaluation through normal pregnancy and in pregnancies complicated by preeclampsia. Acta Obstet. Gynecol. Scand. 2010, 89, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Meinila, J.; Koivusalo, S.B.; Valkama, A.; Rono, K.; Erkkola, M.; Kautiainen, H.; Stach-Lempinen, B.; Eriksson, J.G. Nutrient intake of pregnant women at high risk of gestational diabetes. Food Nutr. Res. 2015, 59. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Bock, C.; Wetzel, M.; Maul, H.; Loerbroks, A. The prevalence of gestational diabetes in advanced economies. J. Perinat. Med. 2012, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Rieck, S.; Kaestner, K.H. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010, 21, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Brewster, S.; Zinman, B.; Retnakaran, R.; Floras, J.S. Cardiometabolic consequences of gestational dysglycemia. J. Am. Coll. Cardiol. 2013, 62, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Tong, J.; Wallace, T.M.; Kodama, K.; Shofer, J.B.; Heckbert, S.R.; Boyko, E.J.; Fujimoto, W.Y.; et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006, 29, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Shah, B.R. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: A population-based cohort study. Can. Med. Assoc. J. 2009, 181, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.C.; Schleyhahn, F.T.; Huffman, D.G.; Amankwah, K.S. Gestational diabetes diagnostic criteria: Long-term maternal follow-up. Am. J. Obstet. Gynecol. 1995, 172, 621–625. [Google Scholar] [CrossRef]
- Feig, D.S.; Zinman, B.; Wang, X.; Hux, J.E. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. Can. Med. Assoc. J. 2008, 179, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Gestational diabetes mellitus. Diabetes Care 2000, 23 (Suppl. 1), S77–S79. [Google Scholar]
- Karoli, R.; Siddiqi, Z.; Fatima, J.; Shukla, V.; Mishra, P.P.; Khan, F.A. Assessment of noninvasive risk markers of subclinical atherosclerosis in premenopausal women with previous history of gestational diabetes mellitus. Heart Views 2015, 16, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lauenborg, J.; Mathiesen, E.; Hansen, T.; Glumer, C.; Jorgensen, T.; Borch-Johnsen, K.; Hornnes, P.; Pedersen, O.; Damm, P. The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J. Clin. Endocrinol. Metab. 2005, 90, 4004–4010. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Qi, Y.; Connelly, P.W.; Sermer, M.; Zinman, B.; Hanley, A.J. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J. Clin. Endocrinol. Metab. 2010, 95, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Qi, Y.; Connelly, P.W.; Sermer, M.; Hanley, A.J.; Zinman, B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of low-density-lipoprotein cholesterol and apolipoprotein b in young women: Implications for future cardiovascular risk. J. Clin. Endocrinol. Metab. 2010, 95, 4345–4353. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.M.; Barbosa, F.B.; de Almeida, M.C.; Miranda, P.A.; Barbosa, M.M.; Nogueira, A.I.; Guimaraes, M.M.; Nunes Mdo, C.; Ribeiro-Oliveira, A., Jr. Previous gestational diabetes is independently associated with increased carotid intima-media thickness, similarly to metabolic syndrome—a case control study. Cardiovasc. Diabetol. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Chiang, V.; Pletcher, M.J.; Jacobs, D.R.; Quesenberry, C.P.; Sidney, S.; Lewis, C.E. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: The coronary artery risk development in young adults study. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.I. Glucose tolerance in pregnancy and the long-term risk of cardiovascular disease. Diabetes Res. Clin. Pract. 2009, 85, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fadl, H.; Magnuson, A.; Ostlund, I.; Montgomery, S.; Hanson, U.; Schwarcz, E. Gestational diabetes mellitus and later cardiovascular disease: A swedish population based case-control study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cheng, Y.J.; Beckles, G.L. Cardiovascular disease risk profiles in women with histories of gestational diabetes but without current diabetes. Obstet. Gynecol. 2008, 112, 875–883. [Google Scholar] [CrossRef] [PubMed]
- North, R.A.; McCowan, L.M.; Dekker, G.A.; Poston, L.; Chan, E.H.; Stewart, A.W.; Black, M.A.; Taylor, R.S.; Walker, J.J.; Baker, P.N.; et al. Clinical risk prediction for pre-eclampsia in nulliparous women: Development of model in international prospective cohort. BMJ 2011, 342. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Diaz-Recasens, J.; Griffin, D.R.; Cohen-Overbeek, T.E.; Pearce, J.M.; Willson, K.; Teague, M.J. New doppler technique for assessing uteroplacental blood flow. Lancet 1983, 1, 675–677. [Google Scholar] [CrossRef]
- Duckitt, K.; Harrington, D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ 2005, 330, 565. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, J.S.; Vollebregt, K.C.; de Vrieze, N.; ter Riet, G.; Mol, B.W.; Franx, A.; Khan, K.S.; van der Post, J.A. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: Systematic review and meta-analysis. BMJ 2008, 336, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Wright, D.; Ispas, C.A.; Poon, L.C.; Nicolaides, K.H. Mean arterial pressure in the three trimesters of pregnancy: Effects of maternal characteristics and medical history. Int. J. Gynaecol. Obstet. 2015, 45, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, R.; Rajakulasingam, R.; Memmo, A.; Bhide, A.; Thilaganathan, B. Uterine artery doppler screening for pre-eclampsia: Comparison of the lower, mean and higher first-trimester pulsatility indices. Int. J. Gynaecol. Obstet. 2011, 37, 534–537. [Google Scholar] [CrossRef]
- Allen, R.; Rogozinska, E.; Sivarajasingam, P.; Khan, K.S.; Thangaratinam, S. Effect of diet- and lifestyle-based metabolic risk-modifying interventions on preeclampsia: A meta-analysis. Acta Obstet. Gynecol. Scand. 2014, 93, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Duley, L.; Henderson-Smart, D.J.; Meher, S.; King, J.F. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2007, 2. [Google Scholar] [CrossRef]
- Askie, L.M.; Duley, L.; Henderson-Smart, D.J.; Stewart, L.A.; Group, P.C. Antiplatelet agents for prevention of pre-eclampsia: A meta-analysis of individual patient data. Lancet 2007, 369, 1791–1798. [Google Scholar] [CrossRef]
- Bujold, E.; Roberge, S.; Lacasse, Y.; Bureau, M.; Audibert, F.; Marcoux, S.; Forest, J.C.; Giguere, Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet. Gynecol. 2010, 116, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.M.; Kajantie, E.; Raikkonen, K.; Pesonen, A.K.; Hamalainen, E.; Vainio, M.; Taipale, P.; Laivuori, H. Aspirin in the prevention of pre-eclampsia in high-risk women. BJOG Int. J. Obstet. Gynaecol. 2013, 120. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Nicolaides, K.H.; Demers, S.; Villa, P.; Bujold, E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Int. J. Gynaecol. Obstet. 2013, 41, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Duley, L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Robbins, C.L.; Dietz, P.M.; Bombard, J.; Valderrama, A.L. Gestational hypertension: A neglected cardiovascular disease risk marker. Am. J. Obstet. Gynecol. 2011, 204, e331–e339. [Google Scholar] [CrossRef] [PubMed]
- Ehrenthal, D.B.; Maiden, K.; Rogers, S.; Ball, A. Postpartum healthcare after gestational diabetes and hypertension. J. Womens Health 2014, 23, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Pina, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the american heart association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- Catov, J.M. Pregnancy as a window to cardiovascular disease risk: How will we know? J. Womens Health 2015, 24, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Berks, D.; Hoedjes, M.; Raat, H.; Duvekot, H.J.; Steegers, E.A. (68-or): Effects of lifestyle intervention after complicated pregnancy: Results of the pro-active study. Pregnancy Hypertens. 2015, 5, 36–37. [Google Scholar] [CrossRef]
- Berks, D.; Hoedjes, M.; Raat, H.; Duvekot, J.J.; Steegers, E.A.; Habbema, J.D. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: A literature-based study. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Ratner, R.E.; Christophi, C.A.; Metzger, B.E.; Dabelea, D.; Bennett, P.H.; Pi-Sunyer, X.; Fowler, S.; Kahn, S.E. Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: Effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab. 2008, 93, 4774–4779. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.H.; Hermes, W.; Breimer, A.Y.; van Rijn, B.B.; Koenen, S.V.; Mol, B.W.; Franx, A.; de Groot, C.J.; Koster, M.P. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015, 65, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, A.L.; Verbeek, A.J. Timely assessment of cardiovascular risk after preeclampsia. J. Womens Health 2014, 10, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Janmohamed, R.; Montgomery-Fajic, E.; Sia, W.; Germaine, D.; Wilkie, J.; Khurana, R.; Nerenberg, K.A. Cardiovascular risk reduction and weight management at a hospital-based postpartum preeclampsia clinic. J. Obstet. Gynaecol. Can. 2015, 37, 330–337. [Google Scholar] [PubMed]
- Philis-Tsimikas, A.; Fortmann, A.L.; Dharkar-Surber, S.; Euyoque, J.A.; Ruiz, M.; Schultz, J.; Gallo, L.C. Dulce mothers: An intervention to reduce diabetes and cardiovascular risk in latinas after gestational diabetes. Transl. Behave Med. 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Spaan, J.; Peeters, L.; Spaanderman, M.; Brown, M. Cardiovascular risk management after a hypertensive disorder of pregnancy. Hypertension 2012, 60, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); Regitz-Zagrosek, V.; Blomstrom Lundqvist, C.; Borghi, C.; Cifkova, R.; Ferreira, R.; Foidart, J.M.; Gibbs, J.S.; et al. Esc guidelines on the management of cardiovascular diseases during pregnancy: The task force on the management of cardiovascular diseases during pregnancy of the european society of cardiology (esc). Eur. Heart J. 2011, 32, 3147–3197. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gongora, M.C.; Wenger, N.K. Cardiovascular Complications of Pregnancy. Int. J. Mol. Sci. 2015, 16, 23905-23928. https://doi.org/10.3390/ijms161023905
Gongora MC, Wenger NK. Cardiovascular Complications of Pregnancy. International Journal of Molecular Sciences. 2015; 16(10):23905-23928. https://doi.org/10.3390/ijms161023905
Chicago/Turabian StyleGongora, Maria Carolina, and Nanette K. Wenger. 2015. "Cardiovascular Complications of Pregnancy" International Journal of Molecular Sciences 16, no. 10: 23905-23928. https://doi.org/10.3390/ijms161023905