Social Isolation and Cognitive Function in Later Life: A Systematic Review and Meta-Analysis
Abstract
Background:
There is some evidence to suggest that social isolation may be associated with poor cognitive function in later life. However, findings are inconsistent and there is wide variation in the measures used to assess social isolation.
Objective:
We conducted a systematic review and meta-analysis to investigate the association between social isolation and cognitive function in later life.
Methods:
A search for longitudinal studies assessing the relationship between aspects of social isolation (including social activity and social networks) and cognitive function (including global measures of cognition, memory, and executive function) was conducted in PsycInfo, CINAHL, PubMed, and AgeLine. A random effects meta-analysis was conducted to assess the overall association between measures of social isolation and cognitive function. Sub-analyses investigated the association between different aspects of social isolation and each of the measures of cognitive function.
Results:
Sixty-five articles were identified by the systematic review and 51 articles were included in the meta-analysis. Low levels of social isolation characterized by high engagement in social activity and large social networks were associated with better late-life cognitive function (r = 0.054, 95% CI: 0.043, 0.065). Sub-analyses suggested that the association between social isolation and measures of global cognitive function, memory, and executive function were similar and there was no difference according to gender or number of years follow-up.
Conclusions:
Aspects of social isolation are associated with cognitive function in later life. There is wide variation in approaches to measuring social activity and social networks across studies which may contribute to inconsistencies in reported findings.
INTRODUCTION
Cognitive aging refers to a process in which some decline in cognitive function is observed as a consequence of healthy aging [1, 2]. Cognitive aging is widely considered to be a normal part of healthy aging whereas clinically significant changes in cognitive function are not [3–5]. The trajectory of cognitive aging varies across older people. Some people experience major cognitive decline that may progress to dementia, whereas others experience subtle changes and minor cognitive impairment, consistent with cognitive aging [6–8]. In addition, decline in some cognitive domains, such as memory and executive function, tends to be more age-related whereas decline in other domains, such as language and general knowledge, tends to be less affected by aging [9–12].
In addition to differences in the trajectories of cognitive aging, it has been observed that some older people have considerable brain pathology without exhibiting concomitant declines in cognition [13–15]. Cognitive reserve theory accounts for this discrepancy and for variations in cognitive aging by proposing that individuals with greater cognitive reserve are able to optimize cognitive performance by recruiting differential brain networks or using alternative cognitive strategies when faced with pathology [16, 17]. Protective lifestyle factors have been identified that may contribute to increased cognitive reserve, such as physical exercise, education, occupational complexity, and engaging in cognitive activity [18–20]. As these lifestyle factors are modifiable, interventions aimed at reducing risk and enhancing modifiable protective factors may provide a basis to ameliorate poor cognitive function [21, 22]. Good social connections may also increase cognitive reserve and protect against declining cognitive function [23]. However, compared to other lifestyle factors, the association between social connections and cognitive function is less clear, with conflicting findings [24, 25].
There are several reasons why the association between social connections and late-life cognition may be less well understood. Firstly, studying social concepts is more complex than assessing lifestyle factors such as physical activity or smoking which may be more readily observable and easier to quantify objectively using a standardized approach [26, 27]. The nature of social connections is more challenging to specify and isolate; for example, social connectivity may occur during other activities that provide cognitive stimulation [28]. It is therefore difficult to determine which factors or combination of factors are most beneficial to cognitive health [28].
In addition, there is a wide range of concepts associated with social connections [29]. Some concepts focus on structural aspects, such as social networks, social isolation, and marital or living situation, whereas others, such as social support, are more related to functional aspects of social contexts, and yet others consider the appraisal of social situations and feelings of loneliness [30]. It can be difficult to isolate specific social concepts as all are likely to interact or contribute to an individual’s social context, yet each is conceptually distinct [31]. Although studies often aim to assess one specific social concept, many create measures that combine questions assessing a range of concepts. For example, one study created a measure of social isolation that classified participants as isolated who were living alone, were unmarried, and had low levels of social support [32]. This measure may not accurately reflect social isolation, as living alone and being unmarried do not necessarily mean an individual is isolated [31]. Likewise, although social support can be useful in determining level of social isolation, both concepts have distinct definitions. Social isolation is defined as having few social contacts and low engagement or integration within a wider community [33] whereas social support focuses more on the availability of social contacts on whom the individual can draw for support if required [34]. Therefore, the extent to which a measure assesses social isolation could be disputed. In addition, some studies aim to assess either social activity or social networks, but often create measures that assess both concepts and sometimes also include other social indicators, such as marital status or living situation [35–39], social support [40], or perceptions of feeling understood [41]. Indeed, measures described as assessing one particular concept may contain elements that assess other distinct social concepts. Therefore, measures may not assess social concepts in isolation which may account for between-study inconsistencies regarding the relationship between social connections and cognitive function [42].
Reverse causality is another methodological issue particularly for cross-sectional studies that assess the association between social connections and cognitive function [43]. The nature of social relationships often changes in later life [44, 45] and there is evidence to suggest that people who experience a decline in cognitive and physical health may be less able to maintain their social relationships [46–48]. Therefore, poor social relationships may be a consequence of cognitive decline rather than a cause [49–51]. The risk of reverse causation can be reduced by using longitudinal data, and studies with a longer interval between the baseline assessment of social measures and follow-up of cognitive function are more reliable for inferring the direction of causality [24, 25].
Several previous reviews have considered the relationship between various aspects of social connections and cognitive function, such as social networks [24], loneliness or perceived isolation [52, 53], social activity and engagement [54], marital status, social networks, and social support [25, 54–56]. Each of these reviews reports equivocal findings regarding the association between aspects of social connections and cognitive function from both cross-sectional and longitudinal studies. A recent review uniquely considered the methodological quality of studies, applied meta-analytic techniques, and also considered structural (social activity and size of social networks) and functional (social support, loneliness, and satisfaction with household members) aspects of social relationships [25]. No previous review has focused on social isolation and the association with cognitive function.
Social isolation is defined as a state in which an individual has a minimal number of social contacts and lacks engagement with others and the wider community [33]. Social isolation can be viewed as a continuum, with isolation and a high level of social participation as opposing extremes [57]. Therefore, social isolation can be captured by studies that assess social networks and social activity or engagement [33]. Being socially isolated may be associated with having fewer social contacts, a smaller social network [16, 43], and less engagement in social activity. In turn, this may be associated with fewer opportunities to make new social contacts, thus leading to a smaller social network and increased isolation [31]. From a cognitive reserve perspective, engaging with people in the social network and participating in social activity is cognitively effortful and hence may contribute to building cognitive reserve and enhancing cognitive function [24, 51].
Given that social isolation may be associated with poor cognitive function in later life, we aimed to investigate, through a systematic review and meta-analysis of data from longitudinal cohort studies, the relationship between aspects of social isolation (including social activity and social networks) and cognitive function in community-dwelling older people. We considered studies that assessed cognition using validated measures of global cognition, as these are frequently used, and measures of memory and executive function, as change in these domains is central to the concept of cognitive aging [9]. Finally, given the variation in approaches to measuring social isolation, we aimed to summarize methods used to assess this concept in articles identified by the review.
METHODS
Systematic search strategy
To identify longitudinal articles assessing the relationship between aspects of social isolation and cognitive function in later life, a systematic search was conducted in PsycInfo, CINAHL, PubMed, and AgeLine for English-language publications to 11 October 2016. No date restrictions were imposed. Search terms focused on three areas: 1) aspects of social isolation (e.g, social relationships, social contact, social activity, social engagement), 2) cognitive function (e.g, cognition, cognitive decline, cognitive health), and 3) later life (e.g, older, aging). See Supplementary Table 1 for full details of the search terms. An identical, updated search was conducted in the same databases on 8 January 2018.
Table 1
Characteristics and results of studies included in the review
| Population characteristics | Measures | |||||||
| Author | Country, Study cohort | Study duration in years | N in analysis | Age, M (SD), range in years | Women (%) | Social isolation measure | Cognitive measure | Study quality |
| Aartsen et al. [28] | Netherlands, Longitudinal Aging Study Amsterdam | 6 | 1126 | 68.7 (8.3), 55–85 | 55 | Social activity: church attendance, neighborhood association, helping others | Global cognition: MMSE | 39 |
| Ellwardt et al. [78] | Mean: 6 Maximum: 20 | 2201 | 67.7 (8.27), 54–85 | 54 | Social network: social network size, number of social roles | Global cognition: MMSE | 40 | |
| Klaming et al. [79] | Maximum: 14 | 1966 | 76.2 (6.8), ≥65 | 54 | Social activity: organization membership, leisure activity | Episodic memory: Rey Auditory Verbal Learning Test | 36 | |
| Albert et al. [66]* | USA, Established Populations for Epidemiologic Studies of the Elderly | Range 2–2.5 | 1192 | 74.3 (2.7), 70–79 | 55 | Social network: number of contacts | Global cognition: composite measure of language (Boston naming test), nonverbal and verbal memory (delayed recognition span test), conceptualization (similarities subtest of the WAIS-R), and visuospatial ability (figure copying) | 35 |
| Bassuk et al. [112] | 12 | 710 | NR, ≥65 | 63 | Social network and activity combined: marital status, frequency of social contact, leisure activity, group membership | Global cognition: SPMSQ | 36 | |
| Béland et al. [67]* | Spain, Aging in Legane’s | 6 | 519 | 75.6 (6.9), 65–100 | 58 | Social network: number of relatives, frequency of contact, living arrangement | Global cognition: PCL | 41 |
| Social activity: group membership, leisure activity | ||||||||
| Zunzunegui et al. [51] | 4 | 557 | NR, ≥65 | 47 | Social network: number of contacts, frequency of contact | Global cognition: composite measure of the SPMSQ, the Barcelona test, and short story recall | 40 | |
| Social activity: group membership, social and leisure activity | ||||||||
| Bennett et al. [80] | USA, Rush Memory and Aging Project | NR | 89 | 84.3 (5.6) | 55 | Social network: number of contacts, frequency of interaction | Global cognition: composite measure of episodic memory (immediate and delayed recall, word list memory, recall, and recognition), semantic memory (Boston naming test, verbal fluency, reading test), working memory, (digit span forward and backward, digit ordering), perceptual speed (symbol digit modalities test, number comparison, Stroop test), and visuospatial ability (judgement line orientation and Raven’s standard progressive matrices). | 28 |
| Boyle et al. [68]* | Mean 4.0 (1.58) Range 1–7 | 698 | 80.4 (7.4) | 75 | 35 | |||
| James et al. [72]* | Mean: 4.5 Maximum: 8 | 954 | 78.4 (NR), ≥55 | 74 | 33 | |||
| James et al. [81] | Mean: 5.2 Range 0.4–12.3 | 1138 | 79.6 (7.5), ≥65 | 74 | Social activity: cultural and leisure activity | 35 | ||
| Bielak et al. [82] | Australia, Australian Longitudinal Study of Ageing | Mean: 5.8 Maximum: 15 | 1321 | 77.46 (NR), 65–98 | 49 | Social activity: group social activity, interaction with friends and family | Immediate episodic memory: Boston naming test Delayed episodic memory: Boston naming test | 39 |
| Giles et al. [83]2 | Maximum: 15 | 706 | 78.6 (5.7), ≥70 | 32 | Social network: number of contact, living arrangement, frequency of contact, existence of confidant | Episodic memory: recall test | 37 | |
| Brown et al. [92]1 | Canada, Victoria Longitudinal Study | Maximum 18 | 977 | 68.6 (6.7), 55–85 | 63 | Social activity: leisure and cultural activity, volunteer work, visiting friends and relatives, organization membership | Memory: list learning and recall Executive function: similarities fluency task | 38 |
| Brown et al. [69]* | Maximum: 18 | 755 | 68.3 (7.0), NR | 65 | 36 | |||
| Small et al. [75]* | Mean: 9.3 Maximum: 12 | 952 | 68.6 (6.7), 55–94 | 63 | Episodic memory: word and story recall | 39 | ||
| Semantic memory: fact recall and vocabulary | ||||||||
| Ertel et al. [70]* | USA, Health and Retirement Study | 6 | 16638 | 64.5 (0.08), 51–99 | 58 | Social network and activity combined: marital status, volunteer work, visiting friends, family, and neighbors | Memory: immediate and delayed recall | 38 |
| Nelson et al. [36] | Maximum: 12 | 203 | NR, ≥50 | 59 | Memory: TICS-M | 35 | ||
| Global cognition: TICS-Mental status | ||||||||
| Glei et al. [84] | Taiwan, Study of Health and Living Status of the Elderly in Taiwan | Maximum: 7 | 2387 | 71.8 (5.2), 64–94 | 44 | Social network: marital status, number of contacts, frequency of contact | Global cognition: SPMSQ | 39 |
| Social activity: volunteer work, leisure activities, visiting friends and relatives, organization membership | ||||||||
| Hsu et al. [71] | 6 | 3302 | NR, ≥60 | 44 | Social activity: paid/unpaid work, organization membership, social club | Global cognition: SPMSQ | 35 | |
| Yen et al. [85] | 10 | 1142 | 69.8 (4.9), ≥64 | 59 | Social activity: volunteer work, participating in group activity | Global cognition: SPMSQ | 37 | |
| Haslam et al. [86] | UK, English Longitudinal Study of Ageing | Maximum: 4 | 3413 | 62.6 (8.9), 50–99 | 57 | Social activity: relationship quality, frequency of contact, number of close contacts Social network: cultural and leisure activities, group membership | Global cognition: composite measure of orientation (orientation measure from MMSE), immediate and delayed memory (immediate and delayed verbal learning task), prospective memory (remembering to carry out a previous instruction), and verbal fluency (category recall) | 38 |
| Shankar et al. [87] | 4 | 6034 | 65.6 (9.5), ≥50 | 55 | Social network and activity combined: marital status, frequency of contact with family and friends, organization membership, leisure activity | Memory: immediate and delayed word recall Executive function: verbal fluency test | 40 | |
| Hill et al. [35] | USA, Hispanic Established Populations for Epidemiologic Study of the Elderly | 8 | 2472 | 72.3 (6.1), 65–107 | 58 | Social network and activity combined: marital status, living arrangement, church attendance, frequency of contact with family | Global cognition: MMSE | 37 |
| Howrey et al. [88] | Maximum: 18 | 2767 | 73.2 (6.5), ≥65 | 58 | Social activity: church attendance | Global cognition: MMSE | 38 | |
| Li &Zhang [40] | China, Chinese Longitudinal Healthy Longevity Survey | 7 | 4190 | 77.6 (9.4), 64–114 | 54 | Social network and activity combined: marital status, number of close children, social support, leisure activity, social groups | Global cognition: MMSE | 39 |
| Zhang [77]* | 2 | 3867 | 83.8, 90–105 | 59 | Social network: marital status, number of children who visit regularly | Global cognition: MMSE | 38 | |
| Marioni et al. [74]* | France, PAQUID | Maximum: 20 | 3653 | 75.3 (6.8), ≥65 | 58 | Social activity: group membership, visits from family and friends | Global cognition: MMSE | 38 |
| Marioni et al. [73]* | Maximum: 20 | 2854 | 77.0 (6.8) | 59 | Social network: number of contacts | Global cognition: MMSE | 40 | |
| Stoykova et al. [41] | Mean: 9.2 (6.6) Maximum: 20 | 2052 | 74.6 (6.66), ≥65 | 54 | Social network: number of contacts, satisfaction with relationships, social group membership | Global cognition: MMSE | 40 | |
| McHugh Power et al. [89] | Ireland, Irish Longitudinal Study of Ageing | 2 | 6985 | 63.5 (9.5), 50–80 | 54 | Social activity: social and leisure activities | Global cognition: composite measure of immediate and delayed recall and MMSE | 37 |
| Santini et al. [38] | Median: 2 years Range: 16–40 months | 6098 | 6.3 (9.2), ≥50 | 52 | Social network and activity combined: marital status, number of contacts, frequency of contact, church attendance, group membership | Global cognition: MMSE | 39 | |
| Niti et al. [90] | Singapore, Singapore Longitudinal Aging Studies | Median: 1.5 Range 1-2 | 1635 | 66.0 (7.3), 55–93 | 65 | Social activity: cultural and leisure activities | Global cognition: MMSE | 39 |
| Schwingel et al. [91] | 2 | 1754 | NR, ≥55 | NR | Social activity: volunteering/paid work | Global cognition: MMSE | 39 | |
| Thomas et al. [96] | USA, American Changing Lives Survey | 3 | 1642 | Men: 69.4, 60–92 Women: 70.4, 60–95 | 67 | Social activity: frequency of social contact, volunteer work, group membership, church attendance | Global cognition: SPMSQ | 39 |
| Thomas et al. [76]* | Maximum: 16 Average: 2.6 | 1667 | 70.1 (NR), ≥60 | 67 | Global cognition: SPMSQ | 37 | ||
| Barnes et al. [111] | USA, Chicago Health and Aging Project | Mean: 5.3 Maximum: 6 | 3899 | 73.9 (6.5), ≥65 | 62 | Social network: number of contacts, frequency of contact | Global cognition: composite measure of episodic memory (immediate and delayed recall), perceptual speed (symbol digit modalities test), and the MMSE | 40 |
| Social activity: cultural and leisure activities, paid/ volunteer work | ||||||||
| Barnes et al. [99] | USA, Study of Osteoporotic Fractures | Maximum: 15 | 9704 | 71.7 (5.3), 65–99 | 100 | Social network: Lubben Social Network Scale (LSNS) | Global cognition: Modified MMSE | 37 |
| Bosma et al. [100] | Netherlands, Longitudinal Maastricht Aging Study | 3 | 818 | NR, 49–81 | NR | Social activity: organizational membership | Global cognition: MMSE | 40 |
| Bourassa [93] | Europe**, Survey of Health, Ageing, and Retirement in Europe | 6 | 19832 | 64.4 (10.0), ≥50 | 54 | Social activity: volunteer work, leisure activity, group membership | Memory: immediate and delayed word recall Executive function: category fluency task | 40 |
| Brown et al. [92]1 | Sweden, Origins of Variance in the Oldest-Old (OCTO) | Maximum: 8 | 524 | 83.2 (2.9), ≥80 | 66 | Social activity: number of social contacts | Memory: immediate recall | 38 |
| USA, Long Beach Longitudinal Study | Maximum: 9 | 565 | 73.8 (9.1), ≥55 | 49 | Social activity: frequency of social contact, volunteer work, leisure activity, visiting friends and family | Memory: immediate recall | ||
| USA, Seattle Longitudinal Study | Maximum 21 | 1657 | 67.1 (8.2), ≥55 | 52 | Executive function: word fluency test | |||
| Gallucci et al. [101] | Italy, Treviso Longeva | 7 | 309 | 79.1 (9.7), 70–105 | 60 | Social activity: visiting friends, volunteer work, social groups | Global cognition: MMSE | 37 |
| Ghisletta et al. [113] | Switzerland, Swiss Interdisciplinary Longitudinal Study on the Oldest Old | 5 | 529 | 83.4 (2.6), 80–85 | 52 | Social activity: cultural and leisure activities | Global cognition: composite measure of executive function (category fluency test) and perceptual speed (cross-out test) | 37 |
| Ho et al. [94] | China, Sample of Chinese elderly | 3 | Men: 519 Women: 469 | 77.4 (5.99), ≥70 | 47 | Social network and activity combined: contact with friends, relatives, neighbors, religious attendance, community groups | Global cognition: composite measure of the Clifton Assessment Procedure for the Elderly, MMSE, and the Mental Status Questionnaire | 40 |
| Holtzman et al. [118] | USA, Epidemiologic Catchment Area survey, Baltimore | Mean: 12.4 Maximum: 15 | 341 | 61.3 (6.9), 50–81 | 69 | Social network: living arrangement, frequency of contact | Global cognition: MMSE | 41 |
| Hughes et al. [116] | USA, Charlotte County Healthy Aging Study | Mean: 4.9 Range 4.6–5.3 | 217 | 72.4 (6.2), ≥65 | 52 | Social network: frequency of social contact, number of contacts | Global cognition: Modified MMSE Memory | 40 |
| Iwasa et al. [102] | Japan, Otasha-Kenshin | 5 | 567 | 75.8 (3.5), 70–84 | 50 | Social activity: volunteer work, group social activities | Global cognition: MMSE | 41 |
| Jedrziewski et al. [105] | USA, National Long Term Care Survey | 10 | 927 | NR, ≥65 | 65 | Social activity: frequency of social contact, organization membership, religious attendance | Global cognition: SPMSQ | 40 |
| Kareholt et al. [106] | Sweden, Random samples of the Swedish population | Mean: 22.8 Range: 21–24 | 1643 | 57.4 (NR), 46–85 | 59 | Social activity: visiting/being visited by friends and relatives | Global cognition: MMSE | 40 |
| Katja et al. [95] | Finland, Evergreen Project | 21 | 1181 | NR, 65–84 | 66 | Social activity: cultural and leisure activities, organization membership, volunteer work | Global cognition: Mini-D | 38 |
| Lee et al. [107] | South Korea, Suwon Longitudinal Aging Study | 2 | 977 | 73.0 (5.7), ≥65 | 61 | Social activity: frequency of social contact, leisure and cultural activity | Global cognition: MMSE | 40 |
| Lee &Kim [103] | Korea, Korean Longitudinal Study of Aging | 4 | 1568 | 71.06 (0.12) ≥65 | 46 | Social activity: organization membership, religious attendance Social network: frequency of social contact | Global cognition: MMSE | 40 |
| Leung et al. [64]* | China, Population based community survey of Hong Kong Chinese | 22 months | 505 | 74.5 (7.1), 61–100 | 55 | Social activity: volunteer work, cultural and leisure activity | Global cognition: MMSE | 35 |
| Li &Hsu [98]2 | Taiwan, Taiwan Longitudinal Study of Aging | 4 | 3226 | 62.7 (9.6), ≥65 | 54 | Social activity: volunteer/paid work, organization/group membership | Global cognition: SPMSQ | 38 |
| McGue &Christensen [108] | Denmark, Longitudinal Study of Aging Danish Twins | Maximum: 8 | 70 | 75.7 (5.2), ≥75 | 63 | Social activity: leisure activity, visiting or being visited by friends and family | Global cognition: MMSE Global cognition: composite measure of executive function (verbal fluency), working memory (forward and backward digit span) and memory (immediate and delayed recall) | 39 |
| McHugh Power et al. [117] | Ireland, Community dwelling Irish | 2 | 624 | 72 (6.8), 60–89 | 68 | Social network: Lubben Social Network Scale (LSNS) | Global cognition: MMSE | 39 |
| Monastero et al. [115] | Sweden, Kungsholmen Project | Mean: 3.4 | 718 | 80.4, 75–95 | 74 | Social activity: leisure activity | Global cognition: MMSE | 39 |
| Social network: number of contacts | ||||||||
| Mousavi-Nasab et al. [109] | Sweden, Betula Project | 5 | 794 | 74.1 (7.1), 65–85 | 55 | Social activity: visiting family and friends, cultural and leisure activity | Episodic memory: free and cued recall and recognition Semantic memory: vocabulary and verbal fluency | 38 |
| Obisesan &Gillum [37] | USA, The Third National Health and Nutrition Examination Survey | Mean: 8.5 Range: 6–12 | 5908 | NR, ≥60 | NR | Social network and activity combined: marital status, frequency of social contact, religious attendance, volunteer wok | Global cognition: Short Index of Cognitive Function | 38 |
| Plehn et al. [65]* | USA, Community dwelling Virginia | Mean: 3.6 Range: 3.2–4.3 | 96 | 75.6 (7.9), ≥55 | 78 | Social activity: social subscale from the SELF-scale | Global cognition: composite measure of Mattis Dementia Rating Scale, Fuld object memory evaluation, and MMSE | 37 |
| Seeman et al. [114] | USA, McArthur Studies of Successful Aging | Mean 7.4 | 706 | 74.2, 70–79 | 55 | Social activity: marital status, number of social contacts Social network: social group membership | Global cognition: composite measure of language (Boston naming test), abstraction (similarities subtest of the WAIS-R), spatial ability (copying), delayed spatial recognition, immediate and delayed story recall | 38 |
| Shatenstein et al. [104] | Canada, Nutrition and Cognition Study | 3 | 1208 | 74.2, 67–84 | 53 | Social activity: cultural and leisure activity, community groups | Global cognition: 3MS | 40 |
| Tomioka et al. [97] | Japan, Community dwelling Japanese | 3 | 6093 | 72.8, 65–96 | 55 | Social activity: leisure activity, volunteer work, social groups, organization membership | Global cognition: Cognitive Performance Scale | 41 |
| Van Ness &Kasl [39] | USA, Yale Health and Aging Project | 6 | 1245 | 74.6 (6.9), ≥65 | 58 | Social network and activity combined: marital status, frequency of contact with family and friends, social groups | Global cognition: SPMSQ | 36 |
| Wang et al. [110] | China, Sample of Chinese elderly people | Mean: 4.7 Maximum: 5 | 5437 | 63.4 (NR), ≥55 | 51 | Social activity: visiting friends and family | Global cognition: MMSE | 39 |
| Wang et al. [50] | China, Longitudinal population-based study of Chinese | Mean: 2.4 Range: 2.3–2.6 | 1463 | 71.0 (5.0), ≥65 | 49 | Social activity: visiting or being visited by friends and family, giving advice | Global cognition: CSID Episodic memory: word list learning and recall, and story recall Executive function: token test | 40 |
NR, not reported; MMSE, Mini-Mental State Examination; WAIS-R, Wechsler Adult Intelligence Scale-Revised; SPMSQ, Short Portable Mental Status Questionnaire; PCL, Leganés’ Cognitive Test (Prueba Cognitiva de Leganés); TICS-M, Modified Telephone Interview for Cognitive Status – Memory; TICS-Mental status, Modified Telephone Interview for Cognitive Status – Mental status; CSID, Community Screening Instrument for Dementia; AD, Alzheimer’s Disease. 1This study reports data for four different cohorts: Origins of Variance in the Oldest-Old, Long Beach Longitudinal Study Participants, Seattle Longitudinal Study, and Victoria Longitudinal Study. 2Data for the total sample is reported in all meta-analyses except for the sub-analysis on gender where data for men and women are reported separately. *Data not reported in the meta-analysis. **Austria, Belgium, Czech Republic, Denmark, France, Germany, Greece, Italy, Netherlands, Poland, Sweden, Spain, and Switzerland.
Inclusion criteria
Articles were included if 1) the sample comprised people who were community-dwelling, ≥50 years at baseline, and with no cognitive impairment, 2) measured social isolation in terms of social network/contact and/or social engagement/activity, 3) measured cognitive function, decline, or change using a standardized measure of global cognitive function, memory, or executive function, 4) longitudinal with a minimum of one-year follow-up, providing an assessment of the relationship between social isolation and cognitive outcomes at follow-up, and 5) peer reviewed. Articles that assessed dementia status as an outcome were excluded as they related to dementia diagnosis rather than cognitive function.
Procedure
A flowchart showing how articles were identified is presented in Fig. 1 and includes articles detected in the searches. Titles, abstracts, and full-text articles were screened by two independent reviewers (IEME and RC). Disagreements were resolved in consensus meetings or resolved by reference to a third reviewer (LC). Reference lists of included articles and relevant reviews [24, 25, 52, 54–56] were screened to identify additional articles that were not retrieved in the initial searches. Data extraction included information about study population, assessment of social isolation and cognitive function, statistical methods, and results.
Fig.1
Screening process for including articles.
The methodological quality of included articles was assessed by a single reviewer (IEME) based on the Critical Appraisal Skills Programme checklist for cohort studies and published guidelines [58]. The checklist comprised 14 items covering the following areas: study aims, population, method, measures, results, and analysis (see Supplementary Material 2). Each article received a score ranging from 1 (poor) to 3 (very good) for each item. Scores were summed to provide an overall quality rating for each article. Possible scores range from 14–42 with higher scores indicating greater methodological quality.
Statistical analysis
To investigate the association between social isolation and cognitive function a correlational random effects meta-analysis was conducted using Comprehensive Meta-Analysis 2 [59]. A standardized correlation direction was used, and where necessary the direction was changed to facilitate cross-study comparisons. For articles where r was not reported, data were transformed into r. For articles that reported a specific p value with standardized or unstandardized coefficients, or odds or hazard ratios, the p value was used. For articles that reported unstandardized coefficients, but without reporting a specific p value (e.g, reported p < 0.05), the precise p value was calculated using the formula suggested by Altman and Bland [60]. Articles that reported standardized coefficients were converted into r using the formula suggested by Peterson and Brown [61]. For articles that reported odds or hazard ratios, but did not report a specific p value, an exact p value was calculated using the formula suggested by Altman and Bland [60]. For articles that used latent growth curve models, or made comparisons across groups (e.g, ANCOVA), specific p values reported in the article for these analyses were used. Where p values were given as a range of significance a cautious approach was used in which the value used to calculate the correlation was set at the upper limit of the range (e.g, for p < 0.05 the value was set at p = 0.049). Where exact non-significant p values were not given and there was insufficient information to calculate a p value, r was reported as 0.
Where multiple articles used data from the same cohort and reported findings based on the same social or cognitive measure, the data included in the meta-analysis were selected based on the following hierarchical criteria: 1) data could be extracted for meta-analysis, 2) articles with the most comprehensive measures of social isolation, 3) longest follow-up duration, and 4) largest sample size. The software package was instructed to average the multiple within-article correlations to correct for violations of independence so that all available data could be included in the analysis.
Effect sizes were calculated using the random effects model as the included articles employed different methods of assessing social isolation and cognitive function and included heterogeneous samples of older people. The random effects model estimates and incorporates the magnitude of heterogeneity into the overall estimated effect [62]. Between-article heterogeneity was assessed using an index of inconsistency (I2) [63]. This calculates a percentage of heterogeneity resulting from study differences that is not due to chance; therefore, larger values indicate greater heterogeneity.
Articles identified in the search were grouped based on social measures as assessing either social activity, social networks, or a combination of both, based on how the authors of each article described the social measure assessed. Cognitive measures were grouped as assessing either global cognitive function, memory, or executive function. Several analyses were conducted to assess the relationships between aspects of social isolation and cognition as follows:
(a) All social measures and 1) all cognitive measures, 2) measures of global cognition, 3) memory, and 4) executive function.
(b) Social activity and 1) all cognitive measures, 2) measures of global cognition, 3) memory, and 4) executive function.
(c) Social networks and 1) all cognitive measures, 2) measures of global cognition, and 3) memory.
(d) Measures that assess a combination of social activity and networks and 1) all cognitive measures, 2) measures of global cognition, and 3) memory.
Two further sub-analyses were conducted that considered all social and cognitive measures and assessed:
(e) Gender differences where articles reported findings for men and women separately.
(f) Length of follow-up, divided into 2-3 years, 4–9 years, and 10–24 years follow-up.
We conducted further sub-analyses to assess how specific indicators of social activity and social networks were associated with cognitive function. Finally, we conducted sub-analyses to assess the association between measures of social activity/social networks and specific measures of cognitive function (e.g, the Mini-Mental State Examination: MMSE).
RESULTS
Identification of articles
The search identified 10,384 unique records, of which 621 abstracts were screened, and 208 full-text articles were examined, resulting in 65 articles meeting inclusion for the review. Table 1 summarizes characteristics of each article. Fifty-one articles were included in the meta-analysis.
Excluded articles
Fourteen articles were excluded from the meta-analysis for the following reasons: two articles contained no useable data [64, 65] and twelve articles were based on the same study populations and used the same social and cognitive measures included elsewhere in the review [66–77].
Included articles
Of the 51 articles included in the meta-analysis, seventeen were combined to create eight cohorts of participants as they included the same participants but reported different social and/or cognitive measures as follows: Longitudinal Aging Study Amsterdam [28, 78, 79], Rush Memory and Aging Project [80, 81], Australian Longitudinal Study of Ageing [82, 83], Study of Health and Living Status of the Elderly in Taiwan [84, 85], English Longitudinal Study of Ageing [86, 87], Hispanic Established Populations for Epidemiologic Study of the Elderly [35, 88], Irish Longitudinal Study of Ageing [38, 89], and Singapore Longitudinal Aging Studies [90, 91]. One article reported findings from four cohorts separately [92] and each cohort was included separately in the meta-analysis. One article [93] split and analyzed the sample into two distinct groups so each group was included as an individual study for the purposes of the meta-analysis. Five articles [51, 94–97] reported results for men and women separately. Two articles reported data for men and women together and separately [83, 98]; therefore, combined data were reported in the main analyses while separate data for men and women was included in the gender sub-analysis. One article reported findings for women only [99] and so was included in main analyses and sub-analyses for gender.
Fifty-one cohorts were included in the meta-analysis with a combined sample of 102,035 unique participants. Thirty-four articles assessed social isolation based on social activity or engagement, 15 assessed isolation based on social networks, and 9 articles assessed isolation based on a combination of both social activity and social networks. The duration of follow-up ranged from 2 to 24 years and the sample size of cohorts ranged from 70 to 19,832 participants (Table 1).
Association between social isolation and cognitive function
There was a statistically significant association between social isolation (i.e, social activity and social networks) and cognitive function, although the effect size was small and there was a moderate degree of heterogeneity (Table 2, Fig. 2). When considering specific measures of cognition, social measures were most strongly associated with measures of global cognition, followed by measures of memory, and then executive function. Effect sizes were small and statistically significant but there was considerable heterogeneity for global measures and tests of memory.
Fig.2
Forest plot of the positive association between social measures and cognitive measures, and differences between men and women, and number of years follow-up.
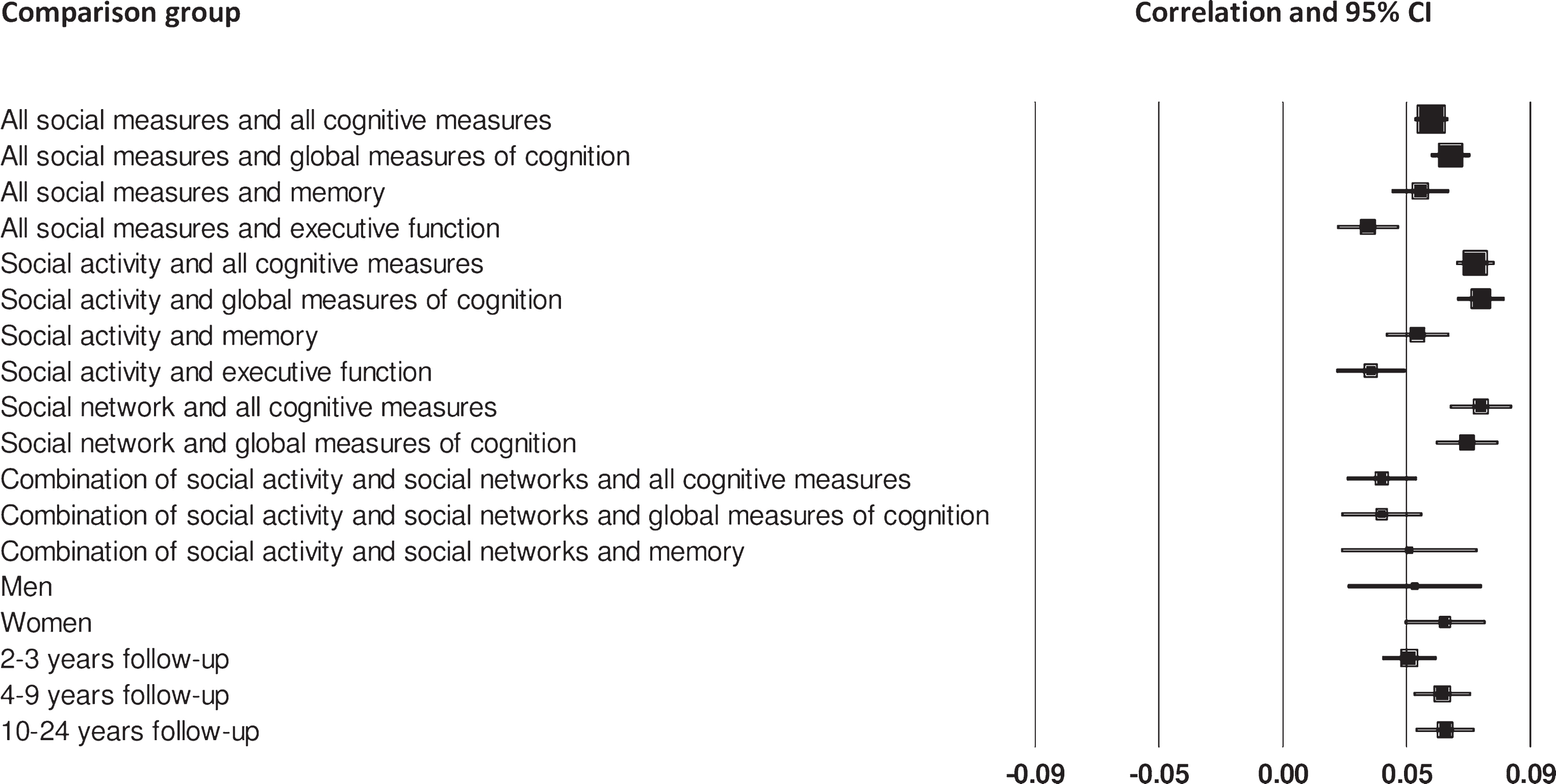
Table 2
Random effects meta-analysis and sub-analyses for aspects of social isolation and cognitive function
| n | k | r | 95% CI | p | Heterogeneity | |||
| Q | Q p | I2 | ||||||
| All social measures | ||||||||
| All cognitive measuresa,b,c | 102,035 | 51 | 0.054 | 0.043, 0.065 | <0.001 | 121.46 | <0.001 | 58.86 |
| Global measuresa,b | 74,933 | 43 | 0.061 | 0.044, 0.079 | <0.001 | 198.12 | <0.001 | 78.80 |
| Memory c | 35,230 | 13 | 0.050 | 0.028, 0.072 | <0.001 | 33.81 | <0.001 | 64.51 |
| Executive function | 30,528 | 7 | 0.031 | 0.015, 0.047 | <0.001 | 9.22 | 0.161 | 34.95 |
| Social activity | ||||||||
| All cognitive measuresa,b,c | 77,954 | 39 | 0.070 | 0.050, 0.089 | <0.001 | 244.89 | <0.001 | 84.48 |
| Global measuresa,b | 51,804 | 31 | 0.072 | 0.048, 0.095 | <0.001 | 194.68 | <0.001 | 84.59 |
| Memoryc | 29,099 | 10 | 0.049 | 0.023, 0.075 | <0.001 | 31.46 | <0.001 | 71.39 |
| Executive function | 24,494 | 6 | 0.032 | 0.011, 0.052 | 0.002 | 9.17 | 0.103 | 45.47 |
| Social network | ||||||||
| All cognitive measuresa | 30,037 | 17 | 0.072 | 0.032, 0.112 | <0.001 | 156.41 | <0.001 | 89.77 |
| Global measuresa | 29,684 | 16 | 0.067 | 0.026, 0.108 | <0.001 | 151.95 | <0.001 | 90.13 |
| Memory | 570 | 2 | 0.107 | –0.041, 0.250 | 0.156 | 2.99 | 0.084 | 66.51 |
| Executive function | – | – | – | – | – | – | – | – |
| Combination of social activity and social networks | ||||||||
| All cognitive measures | 23,783 | 10 | 0.036 | 0.024, 0.049 | <0.001 | 7.32 | 0.604 | 0.00 |
| Global measures | 17,749 | 9 | 0.036 | 0.020, 0.052 | <0.001 | 8.52 | 0.385 | 6.05 |
| Memory | 6,237 | 2 | 0.046 | 0.021, 0.070 | <0.001 | 0.16 | 0.693 | 0.00 |
| Executive function | – | – | – | – | – | – | – | – |
| All social measures and all cognitive measures | ||||||||
| Gender | ||||||||
| Men | 6,448 | 7 | 0.048 | 0.021, 0.074 | <0.001 | 6.49 | 0.371 | 7.48 |
| Women | 17,649 | 8 | 0.059 | 0.028, 0.090 | <0.001 | 18.34 | 0.011 | 61.83 |
| Follow-up time | ||||||||
| 2-3 yearsb | 39,328 | 16 | 0.046 | 0.030, 0.062 | <0.001 | 29.41 | 0.014 | 49.00 |
| 4–9 yearsa,c | 35,374 | 21 | 0.058 | 0.036, 0.080 | <0.001 | 65.86 | <0.001 | 69.63 |
| 10–24 years | 33,393 | 17 | 0.059 | 0.039, 0.078 | <0.001 | 40.09 | <0.001 | 60.09 |
Note: Removing aHaslam et al. [86], bMcHugh Power et al. [89], and cBrown et al OCTO [92] reduced I2 and the effect size r (see Supplementary Material 3 for details).
Engagement in social activity and cognitive function
Thirty-nine cohorts assessed the relationship between social activity and cognitive function (Table 2, Fig. 2). Results suggest that engaging in social activity is significantly associated with better cognitive outcomes on all cognitive measures. When considering each type of cognitive measure separately, social activity was most strongly associated with better cognitive outcomes on global measures of cognition, followed by memory and executive function. Effect sizes were small and statistically significant but there was considerable heterogeneity except for tests of executive function.
Social networks and cognitive function
The association between social networks and cognitive function was assessed in 17 cohorts of participants (Table 2, Fig. 2). The meta-analysis found that larger social networks were significantly associated with better cognitive function when all cognitive measures were combined. This relationship was similar when considering global measures of cognition. Effect sizes were small and statistically significant but with considerable heterogeneity. When measures of memory were considered separately there was no significant association with social networks. While the effect size for the association between social networks and memory was marginally larger than for global measures there were only two cohorts included so this should be treated with some caution, particularly as there was a moderate degree of heterogeneity.
Combination of social activity and social networks and cognitive function
Ten cohorts included measures that assessed both social activity and social networks and the relationship with cognition (Table 2, Fig. 2). The associations between these combined social measures and all measures of cognitive function were statistically significant. The association with global measures was the same as the overall association, and similar for memory. Effect sizes were small and statistically significant, and there was little heterogeneity, suggesting that the effect sizes may be reliable. However, there were only two cohorts included in the memory comparison.
Effect of gender
We next investigated the relationship between social isolation and cognitive function in cohorts that report data for men and women separately. The effect of larger social relationships was similar for men and women. Effect sizes were small and statistically significant with a slight advantage for women (Table 2, Fig. 2), though there was considerably more heterogeneity for women than men.
Effect of follow-up time
Finally, we investigated the association between social isolation and cognitive outcomes over different follow-up times (Table 2, Fig. 2). Effect sizes for each time point were small, statistically significant, but with moderate heterogeneity. Effect sizes were slightly larger for cohorts with a 4–9-and 10–24-year follow-up compared to cohorts with a shorter follow-up of 2-3 years.
Methods of assessing social isolation
The different approaches to assessing social isolation in all articles identified by the systematic review (N = 65) are summarized below. Some indicators of social activity and social networks overlap and were used to assess both concepts.
Social activity/engagement
Fifty-two articles identified by the systematic review assessed social activity. Each of the articles assessed social activity using different indicators of social activity and many articles used more than one indicator within the measure. Twenty-seven articles assessed social and community activities such as attending social or senior citizen clubs, engagement in neighborhood associations, political organizations, and other community groups [28, 39, 41, 51, 64, 67, 71, 74, 79, 81, 82, 84–87, 90, 93–98, 100–104], 21 assessed frequency of visits from or to family, friends, and neighbors [50, 51, 64, 69, 74, 75, 81, 82, 84, 86, 92, 94, 96, 101, 103, 105–110], 23 assessed participation in voluntary or paid work [28, 36–38, 50, 64, 69–71, 81, 84, 85, 91–93, 95–98, 101, 102, 111, 112], and 36 assessed participation in cultural and leisure activities, such as attendance at religious organizations, participating in sport, attending the theatre, museums, exhibitions, eating at restaurants, travelling and overnight trips, attendance to parties, playing games, engaging in hobbies, and reading [28, 35, 37, 38, 40, 51, 64, 67, 69, 71, 75, 79, 81, 84, 86–90, 92–98, 103–105, 107–111, 113, 114]. Ten articles asked about engagement in groups or clubs generally and did not specify the type of groups [38, 40, 69, 71, 92, 105, 111, 112, 114, 115].
Social networks
Twenty-seven articles in the systematic review assessed social networks. Various indicators were used to assess social networks and were included in different combinations within measures across articles. Eighteen articles assessed the number of people within the social network, often using a count of the number of people in the social network [40, 41, 51, 66–68, 72, 73, 78, 80, 83, 84, 99, 111, 114–118], 19 assessed the frequency of interaction with social contacts [35–39, 51, 67, 68, 70, 72, 77, 80, 83, 84, 87, 99, 112, 116–118], 12 assessed marital status [35–40, 70, 77, 84, 87, 112, 114], 3 assessed living arrangements [35, 67, 83], and 3 assessed additional indicators such as satisfaction with social relationships, perception of feeling understood by others, and how many people the participant felt close to [41, 77, 83].
Association between specific indicators of social activity or social networks and cognitive function
Further sub-analyses were conducted to determine whether the different indicators of social activity and social networks could explain heterogeneity or were more associated with measures of cognitive function. There was not enough data to investigate the effects of different social indicators on global cognitive function, memory, and executive function separately, hence we considered the association between specific social indicators and all measures of cognitive function combined. Few articles reported findings for specific indicators separately but where possible sub-analyses were conducted. Social and community activities were described in nine articles [28, 51, 79, 82, 85, 97, 100, 103, 104], frequency of visits from or to, family, friends, and neighbors were described in seven articles [50, 51, 82, 86, 92, 103, 106], voluntary or paid work was described in six articles [28, 85, 91, 95, 97, 98], cultural and leisure activities were described in 12 articles [28, 51, 79, 86, 88–90, 95, 97, 103, 113, 114], social network size was described in six articles [51, 78, 111, 114, 115, 118], and marital status was described in two articles [84, 114]. Heterogeneity was considerably reduced for social and community activities, voluntary or paid work, social network size, and marital status, but remained high for frequency of visits from or to, family, friends, and neighbors, and cultural and leisure activities (Table 3).
Table 3
Random effects sub-analyses for specific indicators of social activity and social network and all measures of cognitive function
| n | k | r | 95% CI | p | Heterogeneity | |||
| Q | Q p | I2 | ||||||
| Social activity | ||||||||
| Social and community activities | 13,903 | 10 | 0.037 | 0.020, 0.054 | <0.001 | 7.79 | 0.555 | 0.00 |
| Frequency of visits from or to family, friends, and neighbors | 10,489 | 8 | 0.074 | 0.029, 0.120 | <0.001 | 33.42 | <0.001 | 79.06 |
| Voluntary or paid work | 14,522 | 8 | 0.043 | 0.024, 0.062 | <0.001 | 8.72 | 0.273 | 19.72 |
| Cultural and leisure activities | 27,120 | 14 | 0.090 | 0.028, 0.151 | 0.005 | 317.48 | <0.001 | 95.91 |
| Social network | ||||||||
| Social network size | 7,716 | 6 | 0.048 | 0.022, 0.074 | <0.001 | 5.75 | 0.332 | 13.00 |
| Frequency of interaction with social contacts | – | – | – | – | – | – | – | – |
| Marital status | 3,093 | 2 | 0.015 | –0.021, 0.050 | 0.413 | 0.08 | 0.774 | 0.00 |
| Living arrangements and proximity to other family | – | – | – | – | – | – | – | – |
Methods of assessing cognitive function
Cognitive function was mostly assessed using measures of global cognitive function. The MMSE was most consistently used across studies [28, 35, 38, 40, 41, 78, 88, 90, 91, 99–103, 106–108, 110, 115, 116, 118] and was the only measure with sufficient data to investigate the association with: all social measures, measures of social activity, measures of social networks, and measures that combined social activity and networks. Heterogeneity was considerably reduced for each group of social measures when the MMSE was the only cognitive measure included in the sub-analyses (Table 4).
Table 4
Random effects sub-analyses for aspects of social isolation and the Mini-Mental State Examination (MMSE)
| n | k | r | 95% CI | p | Heterogeneity | |||
| Q | Q p | I2 | ||||||
| MMSE | ||||||||
| All social measures | 36,587 | 18 | 0.038 | 0.025, 0.050 | <0.001 | 20.91 | 0.230 | 18.71 |
| Social activity | 17,695 | 12 | 0.042 | 0.023, 0.062 | <0.001 | 15.63 | 0.156 | 29.60 |
| Social network | 16,801 | 7 | 0.031 | 0.015, 0.048 | <0.001 | 6.35 | 0.385 | 5.57 |
| Combination of social activity and social networks | 8,695 | 3 | 0.036 | 0.012, 0.061 | 0.003 | 2.54 | 0.282 | 21.11 |
Methodological quality and publication bias
The results of the methodological quality assessment are reported in Table 1. Scores on the quality checklist ranged from 28 to 41 with a mean score of 38.11. Most articles did not use a standardized measure of social isolation and did not consider or compare the characteristics of participants lost to follow-up. There were no articles judged to be of poor methodological quality.
Funnel plots suggest that the results may be slightly overestimated due to publication bias: Egger’s test: b = 1.52, 95% CI: 0.746, 2.285, p < 0.001 (see Supplementary Material 4 for funnel plots).
DISCUSSION
The findings from this systematic review and meta-analysis of longitudinal cohort studies suggest that aspects of social isolation, including low levels of social activity and poor social networks, are significantly associated with poor cognitive function in later life. There was little difference in the effect sizes of reported associations when measures of social isolation were divided into social activity, social networks, and a combination of these two concepts, despite heterogeneous tests of global cognition, memory, and executive function being used. Effect sizes were also similar for men and women, and for number of years follow-up. The effect sizes indicate that having a large social network and engaging in social activity makes a small but statistically significant contribution to preventing poor cognitive function in later life. The size of effect is consistent with a previous review assessing the relationship between poor structural aspects of social relationships and cognitive decline [25]. The small effect size is unsurprising given the range of factors that contribute to maintaining healthy cognitive function [119, 120].
The moderate to high heterogeneity observed in the meta-analysis can be explained by several factors. First, three articles [86, 89, 92] reported effect sizes that were considerably higher than those reported by other included articles. Removing these studies from the meta-analysis reduced heterogeneity considerably and slightly reduced effect sizes. Second, sub-analyses were conducted on articles that assessed cognitive function using the MMSE, hence reducing the variance in assessments of cognitive function. This also considerably reduced heterogeneity and while effect sizes were reduced they remained statistically significant suggesting that global cognition as measured by the MMSE contributes to social activity and social networks.
A wide range of indicators to assess social networks (e.g, number of contacts, frequency of interaction, marital status, living arrangement) and social activity (e.g, attending social groups, visiting family, friends, and neighbors, engaging in voluntary or paid work, participation in cultural or leisure activities) was employed across articles, which may account for the remaining observed heterogeneity [25, 55]. Indeed, further sub-analyses suggested that the heterogeneity may partly be explained by including a range of indicators within measures of social activity and social networks. Heterogeneity was considerably lower for indicators that were specific in nature, such as voluntary or paid work, a count of the number of people within the social network, or social and community activities, which specifically considers social groups and community meetings where the primary outcome is social.
Conversely, heterogeneity was high for cultural and leisure activities, which reflects the diversity of activities that may be included within this indicator and highlights an important methodological issue. Many measures of social activity include questions regarding leisure and cultural activities [79, 81, 86]. These activities are not necessarily social in nature; for example, watching a film or engaging in hobbies may have less social input than visiting friends and family or attending a party. Many cultural and leisure activities present additional demands, for example, playing a game may be both cognitively and socially demanding, and engaging in group sport may be physically, cognitively, and socially demanding [28]. Individual differences may also influence the extent to which an activity is socially demanding [121]. For example, one person may join a bowling club to engage in physical activity, whereas another may enjoy the social aspect of group sports, and a third may gain more cognitive stimulation from thinking strategically about the game. This variation is reflected in the high heterogeneity reported for the specific indicator of leisure and cultural activities and highlights the complexity of assessing social concepts independently from other lifestyle factors and determining the extent of social demand across activities [28, 121]. Heterogeneity was also high for frequency of visits from or to, family, friends, and neighbors. This may be accounted for to some extent by differences in response scales employed across studies, for example, some studies ask about the number of visits received or made within a month [51, 92] while others consider frequency of visits ranging from daily, to yearly/less than yearly [86, 103] and others are more specific and require participants to give the number of hours spent visiting others or being visited [82]. Other studies categorize participants as receiving a high or low number of visits [50] and others are less specific with response categories ranging from never, sometimes, often [106]. The variation in methodological approaches to categorizing ‘frequency’ of visits may account for this heterogeneity.
Few studies reported findings for indicators of social activity or social networks separately and many indicators were included as a range of combinations in measures across studies, which again may account for the heterogeneity observed. Future research should aim to achieve consistency in measures of social concepts and report findings for specific indicators separately. This would enable conclusions regarding the nature of the association between specific aspects of social isolation and cognitive function to be established and inform future cohort or intervention studies [25, 55].
Few randomized controlled trials have investigated the effect of interventions to enhance social connections and cognitive function in later life [54, 122]. In a community-dwelling sample of 250 participants, an intervention to enhance social interaction improved cognitive function and resulted in significant increases in brain volume compared to a control group after 40 weeks [123]. Likewise, increased social activity in 235 lonely people enhanced cognitive function compared to a control group after 12 months [124]. While the effect size for this intervention was moderate the intervention was administered to people who were lonely and so may not be as effective for people who are socially isolated. In addition, a six-week intervention to increase social engagement facilitated by internet video communication was found to improve language based executive functions and psychomotor speed in cognitively healthy older people [125]. This suggests that communication facilitated by the internet may be a cost-effective home-based intervention to enhance social contact and improve cognitive function. Another study reported no beneficial effect of a pilot intervention to enhance social connections on cognitive function [126]. Nonetheless, only five participants were assigned to the social intervention in this study, therefore findings should be treated with caution. Although these studies provide some evidence that interventions to enhance social connections may support the maintenance of healthy cognitive function, both Mortimer et al. [123] and Park et al. [126] report that interventions of physical activity and cognitive activity were more beneficial for cognitive function than interventions to enhance social connections. This evidence, together with the small effect size reported in the meta-analysis may suggest that interventions targeting social isolation alone may be insufficient to reduce poor cognitive function in later life [127].
It is not surprising that the reported association between social isolation and cognitive function is small. There are multiple factors that could impact on trajectories of cognitive decline, including other modifiable lifestyle factors, such as physical exercise, education, occupational complexity, and cognitive activity [119, 120]. It is likely that a range of lifestyle factors, such as cognitive, social, and physical activity, contribute to the maintenance of healthy cognitive function [24, 49]. Cognitive reserve theory suggests that a combination of lifestyle factors across the lifespan contributes to enhancing cognitive reserve and hence maintaining healthy cognitive function [16]. Therefore, diverse environments and activities that increase cognitive stimulation through supporting a range of protective lifestyle factors may be most suitable to build cognitive reserve [54]. The lifestyle factors underpinning cognitive reserve are potentially amenable to change and hence may provide a basis for preventative intervention [21, 22]. This is supported by findings from a recent randomized controlled trial that suggests multi-domain interventions may be most appropriate for the maintenance of cognitive function [127]. Given the small effect sizes reported in the meta-analysis, an intervention to reduce social isolation may be most effective when implemented within a wider intervention that combines a range of lifestyle factors to enhance cognitive reserve [54, 127, 128].
Consistent with the present review, it has been found that poorer social relationships increase the risk of dementia [24, 54, 56, 129, 130]. Individual differences are observed in the expression of healthy cognitive aging and cognitive decline and progression to dementia [6–8]. In line with cognitive reserve theory, differences in trajectories may partly be explained by lifestyle factors [1, 16, 20]. The present review identifies social isolation, as determined by low engagement in social activity and smaller social networks, as a risk factor for poor cognitive function in later life. Future work investigating how integrating interventions to enhance social activity and social networks within multi-domain trials to prevent or delay poor cognitive function and hence progression to dementia is paramount [131]. This is particularly important given that an average delay of two years in the onset of dementia could reduce the worldwide prevalence by 22.8 million cases by 2050 [132].
Among the key strengths of this review, the comprehensive search included several concepts that are associated with social isolation. This enabled us to compare associations between different aspects of social isolation and cognition, including social activity and social networks both overall and separately. We consider the effects that different aspects of social isolation may have on global cognitive function and the specific cognitive domains of memory and executive function [133]. Although fewer studies assessed memory and executive function we found evidence that social isolation is associated with these specific cognitive domains. In addition, we excluded articles reporting findings from cross-sectional data to reduce the risk of reverse causality and enhance the reliability of findings in terms of causality [25, 46]. Only one previous review has used meta-analytic techniques to consider how aspects of social relationships may be associated with cognitive function [25]. We extend this review by considering aspects of social isolation and the association with cognitive function, as well as investigating gender differences in longitudinal studies. Considering gender differences is particularly important given that women may be more likely to engage in frequent social activity and are more likely to maintain close relationships and wider social networks than men [50, 97, 134–136]. Although we report a small association, this still reflects the benefits of social integration on cognitive function in later life for both men and women and is consistent with the findings of Kuiper et al. [25].
Some limitations of the review need to be addressed. First, there was considerable between-article heterogeneity. Additional analysis suggests that this may partly be accounted for by the differences in methodological approaches and range of indicators used to assess social concepts and cognitive function [25] and that other lifestyle factors may contribute to the maintenance of cognitive health [119, 120]; however, this limits our ability to draw definite conclusions regarding the nature of the association. There was evidence of a possible publication bias therefore the observed effect size may be slightly inflated. Studies with a larger sample size and that report a significant association between social relationships and cognitive function are more likely to be reported [137–139] which may account for the publication bias found in the meta-analysis. Including grey literature may have reduced this bias; however, grey literature tends to include studies with small samples and a number of large studies were included in the review that reported statistically non-significant findings. There are large differences in the number of years follow-up across articles which makes it difficult to compare findings. However, findings suggest that there were similar effect sizes irrespective of follow-up duration. An additional limitation applicable to most later life social isolation research is that although socially isolated older people are not uncommon, this group is particularly difficult to engage in research [55]. Therefore, people who are more extremely isolated may be underrepresented in studies that assess the association between social isolation and cognitive function and hence the effect size may be larger than that which we report. Finally, methodological quality was assessed by one reviewer, which may have influenced the methodological quality ratings. However, the ratings were based on standardized criteria and none of the studies were judged to be of poor quality.
We have demonstrated that in later life larger social networks and engagement in social activity are associated with better cognitive function. The reported association was small, which may be attributed to the methodological issues associated with assessing social concepts and the fact that social connections is only one of many factors that influence cognitive function over time. Future studies would benefit from using standardized measures to assess specific social concepts independently. In addition, more randomized controlled trials that assess the effectiveness of interventions to enhance social connections in later life should be conducted to determine whether this may improve cognitive function. This may further help to clarify the nature of the association between social connections and cognitive function in later life.
ACKNOWLEDGMENTS
We are grateful to the Alzheimer’s Society for funding a PhD scholarship for Isobel Evans to complete this work through the following grant: Transdisciplinary training for dementia research in CFAS (The Alzheimer’s Society CFAS Doctoral Training Centre). 2015–2018. AS-DTC-2014-027.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0501r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180501.
REFERENCES
[1] | Liverman CT , Yaffe K , Blazer DG ((2015) ) Cognitive aging: Progress in understanding and opportunities for action. Committee on the Public Health Dimensions of Cognitive Aging; Board on Health Sciences Policy; Institute of Medicine; Blazer DG, Yaffe K, Liverman CT, eds. National Academies Press, Washington, DC. |
[2] | Rabbitt P , Diggle P , Smith D , Holland F , McInnes L ((2001) ) Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia 39: , 532–543. |
[3] | Christensen H ((2001) ) What cognitive changes can be expected with normal ageing? Aust NZJ Psychiatry 35: , 768–775. |
[4] | Deary IJ , Gow AJ , Taylor MD , Corley J , Brett C , Wilson V , Campbell H , Whalley LJ , Visscher PM , Porteous DJ , Starr JM ((2007) ) The Lothian Birth Cohort 1936: A study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr 7: , 28. |
[5] | Stephan B , Matthews , FE , McKeith IG , Bond J , Brayne C ((2007) ) Early cognitive change in the general population: How do different definitions work? J Am Geriat Soc 55: , 1534–1540. |
[6] | Royall DR , Palmer R , Chiodo , LK , Polk MJ ((2005) ) Normal rates of cognitive change in successful aging: The freedom house study. J Int Neuropsych Soc 11: , 899–909. |
[7] | Wilson RS , Beckett LA , Barnes LL , Schneider JA , Bach J , Evans DA , Bennett DA ((2002) ) Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 17: , 179–193. |
[8] | Salthouse TA ((2010) ) Selective review of cognitive aging. J Int Neuropsych Soc 16: , 754–760. |
[9] | Deary IJ , Corley J , Gow AJ , Harris SE , Houlihan LM , Marioni RE , Penke L , Rafnsson BS , Starr JM ((2009) ) Age-associated cognitive decline. Br Med Bullet 92: , 135–152. |
[10] | Ferreira N , Owen A , Mohan A , Corbett A , Ballard C ((2015) ) Associations between cognitively stimulating leisure activities, cognitive function and age-related cognitive decline. Int J Geriatr Psychiatry 30: , 422–430. |
[11] | Gunstad J , Paul RH , Brickman AM , Cohen RA , Arns M , Roe D , Lawrence JJ , Gordon E ((2006) ) Patterns of cognitive performance in middle-aged and older adults: A cluster analytic examination. J Geriatr Psychiatry Neurol 19: , 59–64. |
[12] | Huntley J , Corbett A , Wesnes K , Brooker H , Stenton R , Hampshire A , Ballard C ((2018) ) Online assessment of risk factors for dementia and cognitive function in healthy adults. Int J Geriatr Psychiatry 33: , 1–8. |
[13] | Katzman R , Terry R , DeTeresa R , Brown T , Davies P , Fuld P , Renbing X , Peck A ((1988) ) Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23: , 138–144. |
[14] | Mortimer JA , Snowdon DA , Markesbery WR ((2003) ) Head circumference, education and risk of dementia: Findings from the Nun Study. J Clin Exp Neuropsychol 25: , 671–679. |
[15] | Wharton SB , Brayne C , Savva GM , Matthews FE , Forster G , Simpson J , Lace G , Ince PG ((2011) ) Epidemiological neuropathology: The MRC cognitive function and aging study experience. J Alzheimers Dis 25: , 359–372. |
[16] | Stern Y ((2002) ) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsych Soc 8: , 448–460. |
[17] | Valenzuela MJ , Matthews FE , Brayne C , Ince P , Halliday G , Kril JJ , Dalton MA , Richardson K , Forster G , Sachdev PS ((2012) ) Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol Psychiatry 71: , 783–791. |
[18] | Anstey KJ , Cherbuin N , Herath PM ((2013) ) Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prevent Sci 14: , 411–421. |
[19] | Opdebeeck C , Martyr A , Clare L ((2016) ) Cognitive reserve and cognitive function in healthy older people: A meta-analysis. Aging Neuropsychol Cog 23: , 40–60. |
[20] | Stern Y ((2009) ) Cognitive reserve. Neuropsychologia 47: , 2015–2028. |
[21] | Tucker A , Stern Y ((2011) ) Cognitive reserve in aging. Curr Alzheimer Res 8: , 354–360. |
[22] | Kulmala J , Ngandu T , Kivipelto M ((2018) ) Prevention matters: Time for global action and effective implementation. J Alzheimers Dis 64: (s1), S191–S198. |
[23] | Atti AR , Forlani C , De Ronchi D , Palmer K , Casadio P , Dalmonte E , Fratiglioni L ((2010) ) Cognitive impairment after age 60: Clinical and social correlates in the “Faenza Project”. J Alzheimers Dis 21: , 1325–1334. |
[24] | Fratiglioni L , Paillard-Borg S , Winblad B ((2004) ) An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 3: , 343–353. |
[25] | Kuiper JS , Zuidersma M , Zuidema SU , Burgerhof JG , Stolk RP , Oude Voshaar RC , Smidt N ((2016) ) Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. Int J Epidemiology 45: , 1169–1206. |
[26] | Anstey KJ , von Sanden C , Salim A , O’Kearney R ((2007) ) Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. Am J Epidemiol 166: , 367–378. |
[27] | Beydoun MA , Beydoun HA , Gamaldo AA , Teel A , Zonderman AB , Wang Y ((2014) ) Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Pub Health 14: , 643. |
[28] | Aartsen MJ , Smits CH , Van Tilburg T , Knipscheer KC , Deeg DJ ((2002) ) Activity in older adults: Cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol B Psychol Sci Soc Sci 57: , 153–162. |
[29] | Holt-Lunstad J , Smith TB , Layton JB ((2010) ) Social relationships and mortality risk: A meta-analytic review. PLoS Med 7: , e1000316. |
[30] | Antonucci TC ((1990) ) Social supports, and social relationships. In Handbook of Aging and the Social Sciences, Binstock R, George LK, eds. Academic Press, San Diego, pp.205–226. |
[31] | Victor C , Scambler S , Bond J , Bowling A ((2000) ) Being alone in later life: Loneliness, social isolation and living alone. Rev Clin Geront 10: , 407–417. |
[32] | Holwerda TJ , Deeg DJ , Beekman AT , van Tilburg TG , Stek ML , Jonker C , Schoevers RA ((2014) ) Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). J Neurol Neurosurg Psychiatry 85: , 135–142. |
[33] | Nicholson NR Jr ((2009) ) Social isolation in older adults: An evolutionary concept analysis. J Adv Nurs 65: , 1342–1352. |
[34] | Williams P , Barclay L , Schmied V ((2004) ) Defining social support in context: A necessary step in improving research, intervention, and practice. Qual Health Res 14: , 942–960. |
[35] | Hill TD , Burdette AM , Angel JL , Angel RJ ((2006) ) Religious attendance and cognitive functioning among older Mexican Americans. J Gerontol B Psychol Sci Soc Sci 61: , P3–P9. |
[36] | Nelson LA , Noonan CJ , Goldberg J , Buchwald DS ((2013) ) Social engagement and physical and cognitive health among American Indian participants in the health and retirement study. J Cross-Cultural Gerontol 28: , 453–463. |
[37] | Obisesan TO , Gillum RF ((2009) ) Cognitive function, social integration and mortality in a US national cohort study of older adults. BMC Geriatr 9: , 33. |
[38] | Santini ZI , Koyanagi A , Tyrovolas S , Haro JM , Donovan RJ , Nielsen L , Koushede V ((2017) ) The protective properties of Act-Belong-Commit indicators against incident depression, anxiety, and cognitive impairment among older Irish adults: Findings from a prospective community-based study. Exp Gerontol 91: , 79–87. |
[39] | Van Ness PH , Kasl SV ((2003) ) Religion and cognitive dysfunction in an elderly cohort. J Gerontol B Psychol Sci Soc Sci 58: , S21–S29. |
[40] | Li T , Zhang Y ((2015) ) Social network types and the health of older adults: Exploring reciprocal associations. Soc Sci Med 130: , 59–68. |
[41] | Stoykova R , Matharan F , Dartigues JF , Amieva H ((2011) ) Impact of social network on cognitive performances and age-related cognitive decline across a 20-year follow-up. Int Psychogeriat 23: , 1405–1412. |
[42] | Gow AJ , Mortensen EL ((2016) ) Social resources and cognitive ageing across 30 years: The Glostrup 1914 Cohort. Age Ageing 45: , 480–486. |
[43] | Hultsch DF , Hertzog C , Small BJ , Dixon RA ((1999) ) Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 14: , 245–263. |
[44] | Green AF , Rebok G , Lyketsos CG ((2008) ) Influence of social network characteristics on cognition and functional status with aging. Int J Geriatr Psychiatry 23: , 972–978. |
[45] | Wrzus C , Hänel M , Wagner J , Neyer FJ , ((2013) ) Social network changes and life events across the life span: A meta-analysis. Psychol Bull 139: , 53–80. |
[46] | Aartsen MJ , Van Tilburg T , Smits CH , Knipscheer KC ((2004) ) A longitudinal study of the impact of physical and cognitive decline on the personal network in old age. J Soc Personal Relation 21: , 249–266. |
[47] | Cornwell EY , Waite LJ ((2009) ) Social disconnections, perceived isolation, and health among older adults. J Health Soc Behav 50: , 31–48. |
[48] | Steptoe A , Shankar A , Demakakos P , Wardle J ((2013) ) Social isolation, loneliness, and all-cause mortality in older men and women. Proc Nat Acad Sci USA 110: , 5797–5801. |
[49] | Rizzuto D , Fratiglioni L ((2014) ) Lifestyle factors related to mortality and survival: A mini-review. Gerontol 60: , 327–335. |
[50] | Wang HX , Jin Y , Hendrie HC , Liang C , Yang L , Cheng Y , Unverzagt FW , Ma F , Hall KS , Murrell Ping Li JR , Bian JPJ , Gao S , Kritchevsky S ((2013) ) Late life leisure activities and risk of cognitive decline. J Gerontol A Biomed Sci Med Sci 68: , 205–213. |
[51] | Zunzunegui MV , Alvarado BE , Del Ser T , Otero A ((2003) ) Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. Alzheimers Dement 58: , S93–S100. |
[52] | Cacioppo JT , Hawkley LC ((2009) ) Perceived social isolation and cognition. Trends Cog Sci 13: , 447–454. |
[53] | Boss L , Kang DH , Branson S ((2015) ) Loneliness and cognitive function in the older adult: A systematic review. Int Psychogeriat 27: , 541–553. |
[54] | Wang HX , Xu W , Pei JJ ((2012) ) Leisure activities, cognition and dementia. Biochim Biophys Acta 1822: , 482–491. |
[55] | Kelly ME , Duff H , Kelly S , Power JEM , Brennan S , Lawlor BA , Loughrey DG ((2017) ) The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Rev 6: , 259. |
[56] | Williams JW , Plassman BL , Burke J , Holsinger T , Benjamin S ((2010) ) Preventing Alzheimer’s disease and cognitive decline. Evidence Report 193: , 1–727. |
[57] | de Jong Gierveld J , Havens B ((2004) ) Cross-national comparisons of social isolation and loneliness: Introduction and overview. Can J Aging 23: , 109–113. |
[58] | Downs SH , Black N ((1998) ) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiology Community Health 52: , 377–384. |
[59] | Borenstein M , Hedges LV , Higgins JPT , Rothstein HR ((2005) ) Comprehensive meta-analysis, version 2. Biostat, Engelwood, CO. |
[60] | Altman DG , Bland MB ((2011) ) How to obtain the p value from a confidence interval. BMJ 343: , d2304. |
[61] | Peterson RA , Brown SP ((2005) ) On the use of beta coefficients in meta-analysis. J App Psychol 90: , 175–181. |
[62] | DerSimonian R , Kacker R ((2007) ) Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials 28: , 105–114. |
[63] | Higgins JP , Thompson SG , Deeks JJ , Altman DG ((2003) ) Measuring inconsistency in meta-analyses. BMJ 327: , 557–560. |
[64] | Leung GTY , Fung AWT , Tam CWC , Lui VWC , Chiu HFK , Chan WM , Lam LCW ((2011) ) Examining the association between late-life leisure activity participation and global cognitive decline in community-dwelling elderly Chinese in Hong Kong. Int J Geriatr Psychiatry 26: , 39–47. |
[65] | Plehn K , Marcopulos BA , McLain CA ((2004) ) The relationship between neuropsychological test performance, social functioning, and instrumental activities of daily living in a sample of rural older adults. Clin Neuropsychol 18: , 101–113. |
[66] | Albert MS , Jones K , Savage CR , Berkman L , Seeman T , Blazer D , Rowe JW ((1995) ) Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging 10: , 578–598. |
[67] | Béland F , Zunzunegui MV , Alvarado B , Otero A , del Ser T ((2005) ) Trajectories of cognitive decline and social relations. J Gerontol B Psychol Sci Soc Sci 60: , P320–P330. |
[68] | Boyle PA , Buchman AS , Barnes LL , Bennett DA ((2010) ) Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry 67: , 304–310. |
[69] | Brown CL , Robitaille A , Zelinski EM , Dixon RA , Hofer SM , Piccinin AM ((2016) ) Cognitive activity mediates the association between social activity and cognitive performance: A longitudinal study. Psychol Aging 31: , 831–846. |
[70] | Ertel KA , Glymour MM , Berkman LF ((2008) ) Effects of social integration on preserving memory function in a nationally representative US elderly population. Am J Pub Health 98: , 1215–1220. |
[71] | Hsu HC ((2007) ) Does social participation by the elderly reduce mortality and cognitive impairment?. Aging Ment Health 11: , 699–707. |
[72] | James BD , Boyle PA , Buchman AS , Barnes LL , Bennett DA ((2011) ) Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry 19: , 961–969. |
[73] | Marioni RE , Proust-Lima C , Amieva H , Brayne C , Matthews FE , Dartigues JF , Jacqmin-Gadda H ((2014) ) Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. Eur J Epidemiology 29: , 211–219. |
[74] | Marioni RE , Proust-Lima C , Amieva H , Brayne C , Matthews FE , Dartigues JF , Jacqmin-Gadda H ((2015) ) Social activity, cognitive decline and dementia risk: A 20-year prospective cohort study. BMC Pub Health 15: , 1089. |
[75] | Small BJ , Dixon RA , McArdle JJ , Grimm KJ ((2012) ) Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology 26: , 144–155. |
[76] | Thomas PA ((2011) ) Trajectories of social engagement and limitations in late life. J Health Soc Behav 52: , 430–443. |
[77] | Zhang Z ((2006) ) Gender differentials in cognitive impairment and decline of the oldest old in China. J Gerontol B Psychol Sci Soc Sci 61: , S107–S115. |
[78] | Ellwardt L , Van Tilburg TG , Aartsen MJ ((2015) ) The mix matters: Complex personal networks relate to higher cognitive functioning in old age. Soc Sci Med 125: , 107–115. |
[79] | Klaming R , Annese J , Veltman DJ , Comijs HC ((2017) ) Episodic memory function is affected by lifestyle factors: A 14-year follow-up study in an elderly population. Aging Neuropsychol Cog 24: , 528–542. |
[80] | Bennett DA , Schneider JA , Tang Y , Arnold SE , Wilson RS ((2006) ) The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol 5: , 406–412. |
[81] | James BD , Wilson RS , Barnes LL , Bennett DA ((2011) ) Late-life social activity and cognitive decline in old age. J Int Neuropsych Soc 17: , 998–1005. |
[82] | Bielak AA , Gerstorf D , Anstey KJ , Luszcz MA ((2014) ) Longitudinal associations between activity and cognition vary by age, activity type, and cognitive domain. Psychol Aging 29: , 863–872. |
[83] | Giles LC , Anstey KJ , Walker RB , Luszcz MA ((2012) ) Social networks and memory over 15 years of follow-up in a cohort of older Australians: Results from the Australian Longitudinal Study of Ageing. J Aging Res 2012: , 856048. |
[84] | Glei DA , Landau DA , Goldman N , Chuang YL , Rodriguez G , Weinstein M ((2005) ) Participating in social activities helps preserve cognitive function: An analysis of a longitudinal, population-based study of the elderly. Int J Epidemiology 34: , 864–871. |
[85] | Yen CH , Yeh CJ , Wang CC , Liao WC , Chen SC , Chen CC , L J , Lai T , Lin H , Lee S , Lee MC ((2010) ) Determinants of cognitive impairment over time among the elderly in Taiwan: Results of the national longitudinal study. Arch Geront Geriatr 50: , S53–S57. |
[86] | Haslam C , Cruwys T , Haslam SA ((2014) ) “The we’s have it”: Evidence for the distinctive benefits of group engagement in enhancing cognitive health in aging. Soc Sci Med 120: , 57–66. |
[87] | Shankar A , Hamer M , McMunn A , Steptoe A ((2013) ) Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosomatic Med 75: , 161–170. |
[88] | Howrey TB , Raji AM , Masel MM , Peek KM ((2015) ) Stability in cognitive function over 18 years: Prevalence and predictors among older Mexican Americans. Curr Alzheimer Res 12: , 614–621. |
[89] | McHugh Power J , Tang J , Lawlor B , Kenny RA , Kee F ((2018) ) Mediators of the relationship between social activities and cognitive function among older Irish adults: Results from the Irish longitudinal study on ageing. Aging Ment Health 22: , 129–134. |
[90] | Niti M , Yap KB , Kua EH , Tan CH , Ng TP ((2008) ) Physical, social and productive leisure activities, cognitive decline and interaction with APOE-ɛ4 genotype in Chinese older adults. Int Psychogeriat 20: , 237–251. |
[91] | Schwingel A , Niti MM , Tang C , Ng TP ((2009) ) Continued work employment and volunteerism and mental well-being of older adults: Singapore longitudinal ageing studies. Age Ageing 38: , 531–537. |
[92] | Brown CL , Gibbons LE , Kennison RF , Robitaille A , Lindwall M , Mitchell MB , Shirk SD , Atri A , Cimino CR , Benitez A , MacDonald SWS , Zelinski EM , Willis SL , Warner-Schaie K , Johansson B , Dixon RA , Mungas DM , Hofer SM , Piccin AM ((2012) ) Social activity and cognitive functioning over time: A coordinated analysis of four longitudinal studies. J Aging Res 2012: , 287438. |
[93] | Bourassa KJ , Memel M , Woolverton C , Sbarra D.A ((2017) ) Social participation predicts cognitive functioning in aging adults over time: Comparisons with physical health, depression, and physical activity. Aging Ment Health 21: , 133–146. |
[94] | Ho SC , Woo J , Sham A , Chan SG , Yu AL ((2001) ) A 3-year follow-up study of social, lifestyle and health predictors of cognitive impairment in a Chinese older cohort. Int J Epidemiology 30: , 1389–1396. |
[95] | Katja P , Timo T , Taina R , Tiina-Mari L ((2014) ) Do mobility, cognitive functioning, and depressive symptoms mediate the association between social activity and mortality risk among older men and women? Eur J Ageing 11: , 121–130. |
[96] | Thomas PA ((2011) ) Gender, social engagement, and limitations in late life. Soc Sci Med 73: , 1428–1435. |
[97] | Tomioka K , Kurumatani N , Hosoi H ((2016) ) Social participation and cognitive decline among community-dwelling older adults: A community-based longitudinal study. J Gerontol B Psychol Sci Soc Sci 73: , 799–806. |
[98] | Li CL , Hsu HC ((2015) ) Cognitive function and associated factors among older people in Taiwan: Age and sex differences. Arch Gerontol Geriatr 60: , 196–200. |
[99] | Barnes DE , Cauley JA , Lui LY , Fink HA , McCulloch C , Stone KL , Yaffe K ((2007) ) Women who maintain optimal cognitive function into old age. J Am Geriat Soc 55: , 259–264. |
[100] | Bosma H , van Boxtel MP , Ponds RWHM , Jelicic M , Houx P , Metsemakers J , Jolles J ((2002) ) Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: A reciprocal association? Z Gerontol Geriatr 35: , 575–581. |
[101] | Gallucci M , Mazzuco S , Ongaro F , Di Giorgi E , Mecocci P , Cesari M , Albani D , Forloni GL , Durante E , Gajo GB , Zanardo A , Siculi M , Caberlotto L , Regini C ((2013) ) Body mass index, lifestyles, physical performance and cognitive decline: The “Treviso Longeva (TRELONG)” study. J Nutr Health Aging 17: , 378–384. |
[102] | Iwasa H , Yoshida Y , Kai I , Suzuki T , Kim H , Yoshida H ((2012) ) Leisure activities and cognitive function in elderly community-dwelling individuals in Japan: A 5-year prospective cohort study. J Psychosomatic Res 72: , 159–164. |
[103] | Lee SH , Kim YB ((2016) ) Which type of social activities may reduce cognitive decline in the elderly? A longitudinal population-based study. BMC Geriatr 16: , 165. |
[104] | Shatenstein B , Ferland G , Belleville S , Gray-Donald K , Kergoat M.J , Morais J , Gaudrea P , Payette H , Greenwood C ((2012) ) Diet quality and cognition among older adults from the NuAge study. Exp Gerontol 47: , 353–360. |
[105] | Jedrziewski MK , Ewbank DC , Wang H , Trojanowski JQ ((2014) ) The impact of exercise, cognitive activities, and socialization on cognitive function: Results from the national long-term care survey. Am J Alzheimers Dis Other Demen 29: , 372–378. |
[106] | Kåreholt I , Lennartsson C , Gatz M , Parker MG ((2011) ) Baseline leisure time activity and cognition more than two decades later. Int J Geriatr Psychiatry 26: , 65–74. |
[107] | Lee Y , Kim J , Back JH ((2009) ) The influence of multiple lifestyle behaviors on cognitive function in older persons living in the community. Prevent Med 48: , 86–90. |
[108] | McGue M , Christensen K ((2007) ) Social activity and healthy aging: A study of aging Danish twins. Twin Res Human Genet 10: , 255–265. |
[109] | Mousavi-Nasab SM , Kormi-Nouri R , Nilsson LG ((2014) ) Examination of the bidirectional influences of leisure activity and memory in old people: A dissociative effect on episodic memory. Br J Psychol 105: , 382–398. |
[110] | Wang JYJ , Zhou DHD , Li J , Zhang M , Deng J , Tang M , Gao C , Li J , Lian Y , Chen M ((2006) ) Leisure activity and risk of cognitive impairment: The Chongqing aging study. Neurology 66: , 911–913. |
[111] | Barnes LL , De Leon CM , Wilson RS , Bienias JL , Evans DA ((2004) ) Social resources and cognitive decline in a population of older African Americans and whites. Neurology 63: , 2322–2326. |
[112] | Bassuk SS , Glass TA , Berkman LF ((1999) ) Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med 131: , 165–173. |
[113] | Ghisletta P , Bickel JF , Lövdén M ((2006) ) Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol B Psychol Sci Soc Sci 61: , P253–P261. |
[114] | Seeman TE , Lusignolo TM , Albert M , Berkman L ((2001) ) Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol 20: , 243–255. |
[115] | Monastero R , Palmer K , Qiu C , Winblad B , Fratiglioni L ((2007) ) Heterogeneity in risk factors for cognitive impairment, no dementia: Population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry 15: , 60–69. |
[116] | Hughes TF , Andel R , Small BJ , Borenstein AR , Mortimer JA ((2008) ) The association between social resources and cognitive change in older adults: Evidence from the Charlotte County Healthy Aging Study. J Gerontol B Psychol Sci Soc Sci 63: , P241–P244. |
[117] | McHugh Power J , Lawlor BA , Kee F ((2017) ) Social support mediates the relationships between extraversion, neuroticism, and cognitive function in older adults. Pub Health 147: , 144–152. |
[118] | Holtzman RE , Rebok GW , Saczynski JS , Kouzis AC , Doyle KW , Eaton WW ((2004) ) Social network characteristics and cognition in middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci 59: , P278–P284. |
[119] | Baumgart M , Snyder HM , Carrillo MC , Fazio S , Kim H , Johns H ((2015) ) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11: , 718–726. |
[120] | Clare L , Wu YT , Teale JC , MacLeod C , Matthews F , Brayne C , Woods B , CFAS-Wales study team ((2017) ) Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS Med 14: , e1002259. |
[121] | Toepoel V ((2013) ) Ageing, leisure, and social connectedness: How could leisure help reduce social isolation of older people? Soc Indic Res 113: , 355–372. |
[122] | Yaffe K , Hoang T ((2013) ) Nonpharmacologic treatment and prevention strategies for dementia. Continuum (Minneap Minn) 19: , 372–381. |
[123] | Mortimer JA , Ding D , Borenstein A.R , DeCarli C , Guo Q , Wu Y , Qianhua Z , Chu S ((2012) ) Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. J Alzheimers Dis 30: , 757–766. |
[124] | Pitkala KH , Routasalo P , Kautiainen H , Sintonen H , Tilvis RS ((2011) ) Effects of socially stimulating group intervention on lonely, older people’s cognition: A randomized, controlled trial. Am J Geriatr Psychiatry 19: , 654–663. |
[125] | Dodge HH , Zhu J , Mattek N , Bowman M , Ybarra O , Wild K , Loewenstein DA , Kaye JA ((2015) ) Web-enabled conversational interactions as a method to improve cognitive functions: Results of a 6-week randomized controlled trial. Alzheimers Demen 1: , 1–12. |
[126] | Park DC , Lodi-Smith J , Drew L , Haber S , Hebrank A , Bischof GN , & Aamodt W ((2014) ) The impact of sustained engagement on cognitive function in older adults: The synapse project. Psychol Sci 25: , 103–112. |
[127] | Ngandu T , Lehtisalo J , Solomon A , Levälahti E , Ahtiluoto S , Antikainen R , Bäckman L , Hänninen T , Jula A , Laatikainen T , Lindström J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385: , 2255–2263. |
[128] | Middleton LE , Yaffe K ((2010) ) Targets for the prevention of dementia. J Alzheimers Dis 20: , 915–924. |
[129] | Pillai JA , Verghese J ((2009) ) Social networks and their role in preventing dementia. Ind J Psychiatry 51: , 22–28. |
[130] | Kuiper JS , Zuidersma M , Voshaar RCO , Zuidema SU , van den Heuvel ER , Stolk RP , Smidt N ((2015) ) Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22: , 39–57. |
[131] | Middleton LE , Yaffe K ((2009) ) Promising strategies for the prevention of dementia. Arch Neurol 66: , 1210–1215. |
[132] | Brookmeyer R , Johnson E , Ziegler-Graham K , Arrighi HM ((2007) ) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3: , 186–191. |
[133] | Golden J , Conroy RM , Lawlor BA ((2009) ) Social support network structure in older people: Underlying dimensions and association with psychological and physical health. Psychol Health Med 14: , 280–290. |
[134] | Antonucci TC ((1994) ) A life-span view of women’s social relations. Sage, Thousand Oaks, CA, pp. 239–269. |
[135] | Kavanagh AM , Bentley R , Turrell G , Broom DH , Subramanian SV ((2006) ) Does gender modify associations between self rated health and the social and economic characteristics of local environments? J Epidemiol Community Health 60: , 490–495. |
[136] | Takagi D , Kondo K , Kawachi I ((2013) ) Social participation and mental health: Moderating effects of gender, social role and rurality. BMC Pub Health 13: , 701. |
[137] | Heneghan C , Mahtani KR , Goldacre B , Godlee F , Macdonald H , Jarvies D ((2017) ) Evidence based medicine manifesto for better healthcare: A response to systematic bias, wastage, error and fraud in research underpinning patient care. BMJ 357: , j2973. |
[138] | Williams RJ , Tse T , Harlan WR , Zarin DA ((2010) ) Registration of observational studies: Is it time? Can Med Assoc J 182: , 1638–1642. |
[139] | Altman DG ((2014) ) The time has come to register diagnostic and prognostic research. Clin Chem 60: , 580–582. |



