Abstract
All healthcare systems routinely make resource allocation decisions that trade off potential health gains to different patient populations. However, when such trade-offs relate to the introduction of new, promising health technologies, perceived ‘winners’ and ‘losers’ are more apparent. In recent years, public scrutiny over such decisions has intensified, raising the need to better understand how they are currently made and how they might be improved. The objective of this paper is to critically review and compare current processes for making health technology funding decisions at the regional, state/provincial and national level in 20 countries.
A comprehensive search for published, peer-reviewed and grey literature describing actual national, state/provincial and regional/institutional technology decision-making processes was conducted. Information was extracted by two independent reviewers and tabulated to facilitate qualitative comparative analyses. To identify strengths and weaknesses of processes identified, websites of corresponding organizations were searched for commissioned reviews/evaluations, which were subsequently analysed using standard qualitative methods.
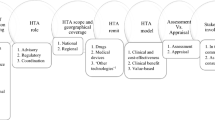
A total of 21 national, four provincial/state and six regional/institutional-level processes were found. Although information on each one varied, they could be grouped into four sequential categories: (i) identification of the decision problem; (ii) information inputs; (iii) elements of the decision-making process; and (iv) public accountability and decision implementation. While information requirements of all processes appeared substantial and decision-making factors comprehensive, the way in which they were utilized was often unclear, as were approaches used to incorporate social values or equity arguments into decisions.
A comprehensive inventory of approaches to implementing the four main components of all technology funding decision-making processes was compiled, from which areas for future work or research aimed at improving the acceptability of decisions were identified. They include the explication of decision criteria and social values underpinning processes.
Similar content being viewed by others
References
Dault M, Lomas J, Barer M. Listening for direction II: a national consultation on health services and policy issues for 2004–2007. Final report. Ottawa (ON): Canadian Health Services Research Foundation, 2004 [online]. Available from URL: http://www.cihr-irsc.gc.ca/e/24509.html [Accessed 2011 Mar 13]
Mitton C, Donaldson C. Health care priority setting: principles, practice and challenges. Cost Eff Resour Alloc 2004; 2 (1): 3
The health of Canadians: the federal role. Final report. Volume six: recommendations for reform. Ottawa (ON): Government of Canada, Standing Senate Committee on Social Affairs, 2002 [online]. Available from URL: http://www.parl.gc.ca/37/2/parlbus/commbus/senate/Com-e/soci-e/rep-e/repoct02vol6-e.htm [Accessed 2011 Mar 13]
Clarke JTR, Amato D, Deber RB. Managing public payment for high-cost, high-benefit treatment: enzyme replacement therapy for Gaucher’s disease in Ontario. CMAJ 2001; 165 (5): 595–6
Commission d’etude sur les services de sante et les services sociaux. Emerging solutions: report and recommendations. Quebec City: Government of Quebec, 2001 [online]. Available from URL: http://msssa4.msss.gouv.qc.ca/en/document/publication.nsf/b640b2b84246d64785256be10060d74/978c5d86bea2903e8525753c00650c1c?OpenDocument [Accessed 2011 Mar 13]
Premier’s Advisory Council on Health. A framework for reform: report of the Premier’s Advisory Council on Health. Edmonton (AB): Government of Alberta, 2001 [online]. Available from URL: http://www.health.alberta.ca/documents/Mazankowski-Report-2001.pdf [Accessed 2011 Mar 13]
Commission on the Future of Health Care in Canada. Building on values: the future of health care in Canada. Final report. Ottawa (ON): Government of Canada, 2002 [online]. Available from URL: http://www.collectionscanada.gc.ca/webarchives/20071122004429/ http://www.hc-sc.gc.ca/english/pdf/romanow/pdfs/hcc_final_report.pdf [Accessed 2011 Mar 13]
Wilking N, Jonsson B. A pan-European comparison regarding patient access to cancer drugs. Stockholm: Karolinska Institutet, 2006 [online]. Available from URL: http://ki.se/content/1/c4/33/52/Cancer_Report.pdf [Accessed 2011 Mar 13]
Bates MJ. Tactics and vocabularies in online searching. In: White HD, Bates MJ, Wilson P, editors. For information specialists: interpretations of reference and bibliographic work. Norwood (NJ): Ablex Publishing, 1992
Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation, 1994
Ramer SL. Site-ation pearl growing: methods and librarianship history and theory. J Med Library Assoc 2005; 93 (3): 397–400 [online]. Available from URL: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1175807 [Accessed 2011 Mar 13]
List of countries by GDP (nominal) per capita. San Francisco (CA): Wikipedia Foundation, Inc., 2010 [online]. Available from URL: http://en.wikipedia.org/wiki/list_of_countries_by_GDP_(nominal)_per_capita [Accessed 2010 Jul 10]
Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2008 [online]. Available from URL: http://www.cochrane.org/resources/handbook/ [Accessed 2011 Mar 13]
Menon D, Stafinski T, Stuart G. Access to drugs for cancer: does where you live matter? Can J Public Health 2005; 96 (6): 454–8
Martin DK, Pater JL, Singer PA. Priority-setting decisions for new cancer drugs: a qualitative case study. Lancet 2001; 358 (9294): 1676–81
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 2004; 13 (5): 437–52
Coye MJ, Kell J. How hospitals confront new technology. Health Aff (Millwood) 2008; 25 (1): 163–74
Daniels N, Sabin J. Limits to health care: fair procedures, democratic deliberation, and the legitimacy problem for insurers. Philos Public Aff 1997; 26 (4): 303–50
Crabtree BF, Miller WL, editors. Doing qualitative research. 2nd ed. Thousand Oaks (CA): Sage Publications, 1999
Noyes J, Popay J, Pearson A, et al. Qualitative research and Cochrane reviews. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2008 [online]. Available from URL: www.cochrane-handbook.org [Accessed 2011 Mar 13]
Lopert R. Evidence-based decision-making within Australia’s pharmaceutical benefits scheme. Issue Brief (Commonw Fund) 2009; 60: 1–13
PBAC submission to the review of health technology assessment in Australia. Canberra (ACT): Pharmaceutical Benefits Advisory Committee, 2009 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/htareview-015 [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. Australia: health policy decision process. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/AustraliaHP.asp [Accessed 2011 Mar 13]
Regulation of medical devices. Canberra (ACT): Australian Government, Department of Health and Ageing, Therapeutic Goods Administration, 2009 [online]. Available from URL: http://www.tga.gov.au/devices/devices.htm [Accessed 2011 Mar 13]
Regulation of therapeutic goods in Australia. Canberra (ACT): Australian Government, Department of Health and Ageing, Therapeutic Goods Administration, 2005 [online]. Available from URL: http://www.tga.gov.au/docs/html/tga/tgaginfo.htm [Accessed 2011 Mar 13]
Morgan SG, McMahon M, Mitton C, et al. Centralized drug review processes in Australia, Canada, New Zealand, and the United Kingdom. Health Aff (Millwood) 2006; 25 (2): 337–47
Healy J, Sharman E, Lokuge B. Australia: health system review. Health systems in transition. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe, 2006 [online]. Available from URL: http://reghealth.anu.edu.au/menus/link_documents/Australia%20E89731.pdf [Accessed 2011 Mar 13]
Haas M, Viney R, Gallego G. Implementing guidelines for reimbursement in Australia: how the PBAC and MSAC use comparative cost-effectiveness. Sydney (NSW): Centre for Health Economics (CHERE)/University of Technology Sydney, 2006 [online]. Available from URL: http://www.hpm.org/Downloads/Symposium_Krakau/Marion_Haas_Australia.pdf [Accessed 2010 Jan 7]
International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world: Australia. Lawrenceville (NJ): ISPOR, 2010 [online]. Available from URL: http://www.ispor.org/PEguidelines/countrydet.asp?c=1&t=2 [Accessed 2011 Mar 13]
PBAC outcomes by meeting. Recommendations made by the PBAC — March 2010. Canberra (ACT): Government of Australia, Department of Health and Ageing, Pharmaceutical Benefits Advisory Committee (PBAC), 2010 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/pbacrec-mar10 [Accessed 2011 Mar 13]
Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee. Version 4.3. Canberra (ACT): Australian Government, Department of Health and Ageing, Pharmaceutical Benefits Advisory Committee, 2008 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/pbacguidelines-index [Accessed 2011 Mar 13]
Alternative arrangements for medicines: other supply arrangements outside the Pharmaceutical Benefits Scheme (PBS). Canberra (ACT): Government of Australia, Department of Health and Ageing, 2010 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/content/lsdp-info [Accessed 2011 Mar 13]
The review of the life saving drugs program. Canberra (ACT): Government of Australia, Department of Health and Ageing, 2010 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/lsdp-info/$File/LSDPreview.pdf [Accessed 2011 Mar 13]
Australian Government Department of Health and Ageing. About the PBS: how do drugs get on the scheme? Canberra (ACT): Government of Australia, Pharmaceutical Benefits Scheme (PBS), 2006 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-general-pbs-phbenbir.htm [Accessed 2011 Mar 13]
Australian Government Department of Health and Ageing. Updated (22 April 2005) questions and answers on new pricing and listing arrangements for generic medicines on the Pharmaceutical Benefits Scheme (PBS). Canberra (ACT): Government of Australia, Pharmaceutical Benefits Scheme (PBS), 2005 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/C96C6E1108952858CA25732B0048D611/$File/qa22april.pdf [Accessed 2011 Mar 13]
Pharmaceutical Benefits Scheme (PBS). Continuation rules for PBS-listed drugs. Canberra (ACT): Government of Australia, Pharmaceutical Benefits Scheme (PBS), 2005 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-general-continuation_rules.htm [Accessed 2011 Mar 13]
The impact of PBS reform: report to the parliament. Canberra (ACT): Government of Australia, Department of Health and Ageing, 2010 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/pbs-reform-report [Accessed 2011 Mar 13]
Advisory Committee on Prescription Medicines (ACPM). Canberra (ACT): Government of Australia, Department of Health and Ageing, Therapeutic Goods Administration, 2010 [online]. Available from URL: http://www.tga.gov.au/committee/acpm.htm [Accessed 2011 Mar 13]
Current MSAC membership. Canberra (ACT): Medical Services Advisory Committee, 2010 [online]. Available from URL: http://www.msac.gov.au/internet/msac/publishing.nsf/content/current-membership-1 [Accessed 2011 Mar 13]
1995 guidelines for the pharmaceutical industry on preparation of submissions to PBAC: including major submissions involving economic analysis. Part 1: role of the PBAC. Canberra (ACT): Government of Australia, Department of Health and Ageing, Pharmaceutical Benefits Advisory Committee (PBAC), 2010 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-general-pubs-guidelines-part1.htm#role [Accessed 2011 Mar 13]
Pharmaceutical Benefits Advisory Committee: PBAC membership. Canberra (ACT): Government of Australia, Department of Health and Ageing, PBAC, 2009 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-general-listing-committee3.htm [Accessed 2011 Mar 13]
PBAC outcomes explained. Canberra (ACT): Government of Australia, Department of Health and Ageing, Pharmaceutical Benefits Advisory Committee (PBAC), 2005 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-general-outcomes.htm [Accessed 2011 Mar 13]
Chalkidou K, Tunis S, Lopert R, et al. Comparative effectiveness research and evidence-based health policy: experience from four countries. Milbank Q 2009; 87 (2): 339–67
Raftery JP. Paying for costly pharmaceuticals: regulation of new drugs in Australia, England and New Zealand. Med J Aust 2008; 188(1): 26–8
Giacomini M. How good is good enough? Standards in policy decisions to cover new health technologies. Healthc Policy 2007; 3 (2): 91–101
Jackson TJ. Health technology assessment in Australia: challenges ahead. Med J Aust 2007; 187 (5): 262–4
Allen Consulting Group. Description of selected health technology assessment processes. Chapter 5: linkages between TGA, MSAC and PDC. Canberra (ACT): Government of Australia, Department of Health and Ageing, 2009 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/allenreport_TOC~allenreport-ch5 [Accessed 2011 Mar 13]
Allen Consulting Group. Description of selected health technology assessment processes: overview of health technology assessment. Canberra (ACT): Government of Australia, Department of Health and Ageing, 2009 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/allenreport_TOC~allenreport-ch1 [Accessed 2011 Mar 13]
Consumers Health Forum of Australia. Information paper: new health technologies, medical devices and prostheses. Canberra (ACT): Consumers Health Forum of Australia, 2007 [online]. Available from URL: http://www.chf.org.au/pdfs/cns/cns-462-new-health-technologies.pdf [Accessed 2011 Mar 13]
Review of health technology assessment in Australia: a discussion paper. Canberra (ACT): Australian Government, Department of Health and Ageing, 2009 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/208F913CD40AD7F9CA2575850080CACD/$File/htadiscussionpaper.pdf [Accessed 2011 Mar 13]
Funding for new medical technologies and procedures: application and assessment guidelines. Canberra (ACT): Medical Services Advisory Committee, 2005 [online]. Available from URL: http://www.health.gov.au/internet/msac/publishing.nsf/Content/D81BE529B98B3DB6CA2575AD0082FD1B/$File/guidelines.pdf [Accessed 2011 Mar 13]
Economics section of the MSAC guidelines. Canberra (ACT): Medical Services Advisory Committee, 2008 [online]. Available from URL: http://www.health.gov.au/internet/msac/publishing.nsf/Content/D81BE529B98B3DB6CA2575AD0082FD1B/$File/Economics%20Glines%20-%20FINAL%20at%20Aug%202008%20-%20endorsed%20MSAC%20ESC%20June%202009.pdf [Accessed 2011 Mar 13]
Medical Services Advisory Committee performance report 2008–09. Canberra (ACT): Medical Services Advisory Committee, 2009 [online]. Available from URL: http://www.health.gov.au/internet/msac/publishing.nsf/Content/9FD4C2646B76FA43CA25768F00221A26/$File/MSAC_Performance%20_Report_2008–09.pdf [Accessed 2011 Mar 13]
Medical Services Advisory Committee. Guidelines for the assessment of diagnostic technologies. Canberra (ACT): Department of Health and Aging, Medical Services Advisory Committee (MSAC), 2005 [online]. Available from URL: http://www.health.gov.au/internet/msac/publishing.nsf/Content/D81BE529B98B3DB6CA2575AD0082FD1B/$File/Diag%20Guidelines%20Sept%202005%20updated%2021%20may%202007.pdf [Accessed 2011 Mar 13]
Healy P, Pugatch M. Theory versus practice: discussing the governance of health technology assessment systems. Stockholm: Stockholm Network, 2009 [online]. Available from URL: http://www.stockholm-network.org/downloads/publications/Theory_versus_Practice.pdf [Accessed 2011 Mar 13]
Wild C. Austria: history of health technology assessment during the past 20 years. Int J Technol Assess Health Care 2009; 25 Suppl. 1: 74–81
Buchholz P. ISPOR global health care systems road map. Austria: pharmaceuticals. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Austria.asp [Accessed 2011 Mar 13]
Pharmaceutical pricing and reimbursement information: Austria. Vienna: European Commission, Health and Consumer Protection Directorate-General and Austrian Ministry of Health, Family and Youth, 2008 [online]. Available from URL: http://ppri.oebig.at/Downloads/Results/Austria_PPRI_2008_Englih_Version.pdf [Accessed 2011 Mar 13]
Habl C, Antony K, Arts D, et al. Surveying, assessing and analysing the pharmaceutical sector in the 25 EU member states: country profiles. Vienna: European Commission, Pharmacoeconomics 2011; 29 (6) Osterreichisches Bundesinstitut fur Gesundheitswesen (OBIG), 2006 [online]. Available from URL: http://ec.europa.eu/competition/mergers/studies_reports/oebig.pdf [Accessed 2011 Mar 13]
Hofmarcher MM, Rack HM, Rohrling G. Austria: health system review. Health Systems in Transition. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe, 2006 [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0009/96435/E89021.pdf [Accessed 2011 Mar 13]
Cleemput I, Van WP, Huybrechts M, et al. Belgian methodological guidelines for pharmacoeconomic evaluations: toward standardization of drug reimbursement requests. Value Health 2009; 12 (4): 441–9
Vinck I, Neyt M, Thiry N, et al. Introduction of emerging medical devices on the market: a new procedure in Belgium. Int J Technol Assess Health Care 2007; 23 (4): 449–54
Health care systems in transition: Belgium. Brussels: European Observatory on Health Care Systems, 2000 [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0003/75126/E71203.pdf [Accessed 2011 Mar 13]
Corens D. Belgium: health system review. Health Systems in Transition. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe, 2007 [online]. Available from URL: http://www.euro.who.int/__data/assets/pdf_file/0007/96442/E90059.pdf [Accessed 2011 Mar 13]
Policies for rare diseases and orphan drugs. KCE reports 112C. Brussels: Belgian Health Care Knowledge Centre, 2009: 35–44 [online]. Available from URL: http://www.kce.fgov.be/Download.aspx?ID=2161 [Accessed 2011 Mar 13]
Procedure for common drug review. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health (CADTH), 2010 [online]. Available from URL: http://www.cadth.ca/media/cdr/process/CDR_Procedure_e.pdf [Accessed 2011 Mar 13]
Common drug review submission guidelines for manufacturers. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health (CADTH), 2010 [online]. Available from URL: http://www.cadth.ca/media/cdr/process/CDR_Submission_Guidelines.pdf [Accessed 2011 Mar 13]
Health technology assessment of medical devices. MEDEC’s position on issues surrounding health technology assessment (HTA) in Canada. Toronto (ON): MEDEC, 2006
Health technology assessment. Fall 2008 position statement. Toronto (ON): MEDEC, 2008 [online]. Available from URL: http://www.medec.org/webfm_send/1219 [Accessed 2011 Mar 13]
Pedersen KM, Christiansen T, Bech M. The Danish health care system: evolution — not revolution — in a decentralized system. Health Econ 2005; 14 Suppl. 1: S41–57
Strandberg-Larsen M, Knudsen MS. ISPOR global health care systems road map. Denmark: pharmaceuticals. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/htaroadmaps/Denmark.asp [Accessed 2011 Mar 13]
Pricing and reimbursement in Denmark. Copenhagen: The Danish Association of the Pharmaceutical Industry, 2007 [online]. Available from URL: http://parno1.ipapercms.dk/LIF/Notater/PricingandreimbursementinDenmark/[Accessed 2011 Mar 13]
The Danish Medicines Agency: reimbursement of medicines. Copenhagen: The Danish Medicines Agency, 2010 [online]. Available from URL: http://laegemiddelstyrelsen.dk/en/topics/statistics,-prices-and-reimbursement/reimbursement-of-medicines [Accessed 2011 Mar 19]
Strandberg-Larsen M, Nielsen MB, Vallgarda S, et al. Denmark: health system review. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe, 2007. Health systems in transition 2007; 9 (6) [online]. Available from URL: http://www.euro.who.int/__data/assets/pdf_file/0004/80581/E91190.pdf [Accessed 2011 Mar 13]
Guidelines for application for general reimbursement of medicinal products. Copenhagen: The Danish Medicines Agency, 2008 [online]. Available from URL: http://laegemiddelstyrelsen.dk/en/service-menu/news/new-guidelines-for-application-for-gener-l-products [Accessed 2011 Mar 13]
Sorenson C. The role of HTA in coverage and pricing decisions: a cross-country comparison. Euro Observer: the Health Policy Bulletin of the European Observatory on Health Systems and Policies 2009; 11 (1): 1–12 [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0019/80335/EuroObserver_spring2009.pdf [Accessed 2011 Mar 13]
Makela M, Roine RP. Health technology assessment in Finland. Int J Technol Assess Health Care 2009; 25 Suppl. 1: 102–7
Sorenson C, Drummond M, Kanavos P. Ensuring value for money in health care: the role of health technology assessment in the European Union. European Observatory on Health Systems and Policies. Observatory Studies Series No. 11. Copenhagen: WHO Regional Office for Europe, 2008 [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0011/98291/E91271.pdf [Accessed 2011 Mar 13]
Pharmaceuticals Pricing Board: the Pharmaceuticals Pricing Board and the secretariat. Helsinki: Ministry of Social Affairs and Health/Social-Och Halsovardsministeriet, 2009 [online]. Available from URL: http://www.stm.fi/en/ministry/boards/pharmaboard/board [Accessed 2011 Mar 13]
Pharmaceuticals Pricing Board: the expert group of the Pharmaceuticals Pricing Board. Helsinki: Ministry of Social Affairs and Health/Social-Och Halsovardsministeriet, 2010 [online]. Available from URL: http://www.stm.fi/en/ministry/boards/pharmaboard/expert [Accessed 2011 Mar 13]
Mossialos E, Srivastava D. Overview of the pharmaceutical system in Finland. In: Mossialos E, Srivastava D. Pharmaceutical policies in Finland: challenges and opportunities. Observatory Studies Series no. 10. Copenhagen: WHO; European Observatory on Health Systems and Policies; Ministry of Social Affairs and Health Finland, 2008: 5–25 [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0020/80651/E91239.pdf [Accessed 2011 Mar 13]
Mossialos E, Srivastava D. Supply-side policies concerning pharmaceuticals. In: Mossialos E, Srivastava D. Pharmaceutical policies in Finland: challenges and opportunities. Observatory Studies Series no. 10. Copenhagen: WHO, European Observatory on Health Systems and Policies, Ministry of Social Affairs and Health Finland, 2008: 79–93 [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0020/80651/E91239.pdf [Accessed 2011 Mar 13]
Financing medical devices in Europe: executive summary. Belgium: The European Health Technology Institute for Socio-Economic Research (EHTI), 2009 [online]. Available from URL: http://www.together4healthinnovation.eu/uploads/Executive%20Summary%20Topic%20I%20Financing%20Medical%20Devices%20in%20Europe.pdf [Accessed 2011 Mar 13]
Medical device assessment in France: guidebook. Paris: Haute Autorite de Sante (HAS), 2009 [online]. Available from URL: http://www.has-sante.fr/portail/upload/docs/application/pdf/2010-03/guide_dm_gb_050310.pdf [Accessed 2011 Mar 13]
Yfantopoulos J. Pharmaceutical pricing and reimbursement reforms in Greece. Eur J Health Econ 2008; 9 (1): 87–97
Surveying, assessing and analysing the pharmaceutical sector in the 25 EU member states. Luxembourg: Osterreichisches Bundesinstitut fur Gesundheitswesen (OBIG) for the European Commission, 2006: 283–96 [online]. Available from URL: http://ec.europa.eu/competition/mergers/studies_reports/oebig.pdf [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. Greece: reimbursement process. Lawrenceville (NJ): ISPOR, 2008 [online]. Available from URL: http://www.ispor.org/htaroadmaps/Greece.asp [Accessed 2011 Mar 13]
Pharmaceutical pricing and reimbursement information: Ireland. Vienna: European Commission, Health and Consumer Protection Directorate-General and Austrian Ministry of Health, Family and Youth, 2007 [online]. Available from URL: http://ppri.oebig.at/Downloads/Results/Ireland_PPRI_2007.pdf [Accessed 2011 Mar 13]
Barry M. Economies in drug usage in the Irish healthcare setting. Dublin: Department of Health and Children, 2009 [online]. Available from URL: http://www.dohc.ie/publications/pdf/economies_drug_usage.pdf?direct=1 [Accessed 2011 Mar 13]
National Centre for Pharmacoeconomics in Ireland. Irish healthcare technology assessment guidelines. Version 1. Dublin: National Centre for Pharmacoeconomics (NCPE) in Ireland, 2000 [online]. Available from URL: http://www.ncpe.ie/contact.php [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. Italy: reimbursement process. Lawrenceville (NJ): ISPOR, 2008 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Italy.asp [Accessed 2011 Mar 13]
Folino-Gallo P, Montilla S, Bruzzone M, et al. Pricing and reimbursement of pharmaceuticals in Italy. Eur J Health Econ 2008; 9 (3): 305–10
Lo Scalzo A, Donatini A, Orzella L, et al. Italy: health system review. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe. Health Systems in Transition 2009; 11 (6) [online]. Available from URL: http://www.euro.who.int/_data/assets/pdf_file/0006/87225/E93666.pdf [Accessed 2011 Mar 13]
Pricing and reimbursement. Rome: Agenzia Italiana del Farmaco (AIFA), 2010 [online]. Available from URL: http://www.agenziafarmaco.it/en/content/pricing-and-reimbursement [Accessed 2011 Mar 13]
Liu GG, Fukuda T, Lee CE, et al. Evidence-based decision-making on medical technologies in China, Japan, and Singapore. Value Health 2009; 12 Suppl. 3: S12–7
International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomics and outcomes research in Asia-Pacific: China, Japan, South Korea, Singapore, Thailand, Pakistan, Malaysia and India. Lawrenceville (NJ): ISPOR, 2006 [online]. Available from URL: http://www.ispor.org/conferences/shanghai0306/Plenary1_tbl.pdf [Accessed 2011 Mar 13]
Fukuda T. The need and development of HTA in Japan. International Health Technology Assessment Symposium; 2008 Aug 11; Taipei [online]. Available from URL: http://www.docser.com/download/Drug_Pricing_in_Japan/aHR0cDovL3d3dy5jZGUub3JnLnR3L3VwbG9hZGZpbGUvZm9ydW1zLzk3MDgxMS80LnBkZg [Accessed 2011 Mar 13]
Pharmaceutical administration and regulations in Japan: health insurance programs and drug pricing in Japan. Tokyo: Japan Pharmaceutical Manufacturers Association (JPMA), 2010: 179–94 [online]. Available from URL: http://www.jpma.or.jp/english/parj/1003.html [Accessed 2011 Mar 13]
Whyte K. PHARMAC not funding some treatments for rare, life-threatening diseases: bosentan as an example. N Z Med J 2005; 118 (1226): U1759
Manning J, Paterson R. “Prioritization”: rationing health care in New Zealand. J Law Med Ethics 2005; 33 (4): 681–97
O’Donnell JL, Smyth D, Frampton C. Prioritizing healthcare funding. Intern Med J 2005; 35 (7): 409–12
Decision-making about new health interventions. Wellington: National Health Committee, 2005 [online]. Available from URL: http://www.nhc.health.govt.nz/moh.nsf/indexcm/nhc-new-health-interventions [Accessed 2011 Mar 13]
Decision-making about new health interventions: a background paper. Wellington: National Health Committee, 2006 [online]. Available from URL: http://www.nhc.health.govt.nz/moh.nsf/pagescm/667/$File/dhb-decisions-new-health-background-paper.pdf [Accessed 2011 Mar 13]
Pharmaceutical Management Agency annual review 2010 [online]. Available from URL: http://www.pharmac.govt.nz/2010/12/15/2010AnnRev.pdf [Accessed 2011 Mar 13]
Medicines strategy consultation document: ‘Towards a medicines strategy’. Wellington: PHARMAC, 2009 [online]. Available from URL: http://www.pharmac.govt.nz/2009/07/10/Submission%20on%20the%20development%20of%20the%20Medicines%20Strategy.pdf [Accessed 2011 Mar 13]
Guidelines for funding applications to PHARMAC. Wellington: PHARMAC, 2009 [online]. Available from URL: http://www.pharmac.govt.nz/2009/12/23/2009-12-23%20-%20PHARMAC%20notification%20of%20final%20Application%20Guidelines.pdf [Accessed 2011 Mar 13]
Operating policies and procedures of the Pharmaceutical Management Agency (PHARMAC). 3rd ed. Wellington: PHARMAC, 2006 Jan [online]. Available from URL: http://www.pharmac.govt.nz/2005/12/22/231205.pdf [Accessed 2011 Mar 13]
Section H of the pharmaceutical schedule (hospital pharmaceuticals). Wellington: PHARMAC, 2010 [online]. Available from URL: http://www.pharmac.govt.nz/Schedule/SectionH [Accessed 2011 Mar 13]
Pharmaceutical schedule. Wellington: PHARMAC, 2010 [online]. Available from URL: http://www.pharmac.govt.nz/Schedule [Accessed 2011 Mar 13]
Prescription for pharmacoeconomic analysis: methods for cost-utility analysis. Final, May 2007. Wellington:PHARMAC, 2007 [online]. Available from URL: http://www.pharmac.govt.nz/2007/06/19/PFPAFinal.pdf [Accessed 2011 Mar 13]
Prescription for pharmacoeconomic analysis: methods for cost-utility analysis. Version 2, July 2006. Wellington: PHARMAC, 2006: 19–25 [online]. Available from URL: http://www.pharmac.govt.nz/2006/07/31/PFPAv2.pdf [Accessed 2011 Mar 13]
How should high cost medicines be funded? Paper for public consultation. Wellington: PHARMAC, 2006 [online]. Available from URL: http://www.pharmac.govt.nz/2006/12/15/HCMConsult.pdf/text [Accessed 2011 Mar 13]
Hansen P. A theoretical review of PHARMAC’s overarching approach to deciding which pharmaceuticals to fund, including high cost ones. Wellington: PHARMAC, 2006 [online]. Available from URL: http://www.pharmac.govt.nz/2006/06/06/HCM2.pdf [Accessed 2011 Mar 13]
Martinussen PE, Hagen TP. Reimbursement systems, organisational forms and patient selection: evidence from day surgery in Norway. Health Econ Policy Law 2009; 4 (Pt 2): 139–58
Norwegian guidelines for pharmacoeconomic analysis in connection with applications for reimbursement. Oslo: Statens legemiddelverk/Norwegian Medicines Agency, 2005 [online]. Available from URL: http://www.legemiddelverket.no/templates/InterPage_25644.aspx [Accessed 2011 Mar 13]
Application standard for acceptance to the drug reimbursement scheme: pursuant to Article 9 of the regulation on reimbursement of crucial drug costs. Oslo: Statens legemiddelverk/Norwegian Medicines Agency, 2005 [online]. Available from URL: http://www.legemiddelverket.no/templates/InterPage_25665.aspx [Accessed 2011 Mar 13]
Pharmaceutical pricing and reimbursement information: Norway. Vienna: Pharmaceutical Pricing and Reimbursement Information, 2008 [online]. Available from URL: http://ppri.oebig.at/Downloads/Results/Norway_PPRI_2008.pdf [Accessed 2011 Mar 13]
Li S-C. Health care system and public sector drug formulary in Singapore. ISPOR Connections 2007 Oct [online]. Available from URL: http://www.ispor.org/news/articles/oct07/hcs.asp [Accessed 2011 Mar 13]
Criteria to determine drugs for the Standard Drug List. Singapore: Singapore Government, Ministry of Health, 2005 [online]. Available from URL: http://www.moh.gov.sg/mohcorp/parliamentaryqa.aspx?id=4690 [Accessed 2011 Mar 13]
Garner S. How decisions on the use of medicines and medical devices are made. Pharmaceutical J 2005; 275 (7364): 254–6
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. Scotland. Lawrenceville (NJ): ISPOR, 2007 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Scotland.asp [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world: Scotland. Lawrenceville (NJ): ISPOR, 2010 [online]. Available from URL: http://www.ispor.org/PEguidelines/countrydet.asp?c=19&t=1 [Accessed 2011 Mar 13]
Guidance to manufacturers for completion of new product assessment form (NPAF). Glasgow: Scottish Medicines Consortium, 2011[online]. Available from URL: http://www.scottishmedicines.org.uk/About_SMC/Latest_News/News_Articles/New_Product_Assessment_Form_NPAF [Accessed 2011 Mar 13]
SMC Evaluation Project Team. An evaluation of how SMC has engaged with its key stakeholders and shaped medicines use across NHS Scotland: summary report. Glasgow: NHS Scotland/Scottish Medicines Consortium, 2008 [online]. Available from URL: http://www.scottishmedicines.org.uk/files/SMC_Evall_FINAL_lores.pdf [Accessed 2011 Mar 13]
Scottish Medicines Consortium. Scottish Medicines Consortium 2010 [online]. Available from URL: http://www.scottishmedicines.org.uk/smc/22.html [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. Spain: pharmaceutical. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Spain.asp [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world: Spain. Lawrenceville (NJ): ISPOR, 2010 [online]. Available from URL: http://www.ispor.org/PEguidelines/countrydet.asp?c=20&t=1 [Accessed 2011 Mar 13]
Appraising treatments which may extend life, at the end of life. London: NICE, 2009 [online]. Available from URL: http://www.nice.org.uk/aboutnice/howwework/devnicetech/endoflifetreatments.jsp [Accessed 2011 Mar 13]
All Wales Medicines Strategy Group: independent review process (IR). Vale of Glamorgan: All Wales Medicines Strategy Group, 2007 [online]. Available from URL: http://www.wales.nhs.uk/sites3/Documents/371/Independent%20Review%20process%20_fina%20for%20website_.pdf [Accessed 2011 Mar 13]
All Wales Medicines Strategy Group: procedure to address complaints relating to scientific disputes via independent review. Vale of Glamorgan: All Wales Medicines Strategy Group, 2007 [online]. Available from URL: http://www.wales.nhs.uk/sites3/Documents/371/Scientific%20disputes%20for%20website%20April07.pdf [Accessed 2011 Mar 13]
All Wales Medicines Strategy Group. Vale of Glamorgan: All Wales Medicines Strategy Group, 2010 [online]. Avail able from URL: http://www.wales.nhs.uk/sites3/home.cfm?orgid=371 [Accessed 2011 Mar 13]
Gallego G, Taylor SJ, Brien JA. Priority setting for high cost medications (HCMs) in public hospitals in Australia: a case study. Health Policy 2007; 84 (1): 58–66
Durán A, Lara JL, van Waveren M. Spain: health system review. In: Bankauskaite V, editor. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe. Health Systems in Transition 2006; 8 (4) [online]. Available from URL: http://www.euro.who.int/en/home/projects/observatory/publications/health-system-profiles-hits/full-list-of-hits/spain-hit-2007 [Accessed 2011 Mar 13]
Stolk EA, De BA, van Halteren AR, et al. Role of health technology assessment in shaping the benefits package in the Netherlands. Expert Rev Pharmacoecon Outcomes Res 2009; 9 (1): 85–94
Stolk P, Schneeweiss S, Leufkens HG, et al. Impact analysis of the discontinuation of reimbursement: the case of oral contraceptives. Contraception 2008; 78 (5): 399–404
Niezen M, De BA, Stolk E, et al. Conditional reimbursement within the Dutch drug policy. Health Policy 2007; 84 (1): 39–50
Stolk EA, Rutten FF. The ‘health benefit basket’ in the Netherlands. Eur J Health Econ 2005 Dec; Suppl.: 53–7
Stolk EA, Poley MJ. Criteria for determining a basic health services package: recent developments in the Netherlands. Eur J Health Econ 2005; 6 (1): 2–7
Postma MJ. Public health economics of vaccines in the Netherlands: methodological issues and applications. J Public Health 2008; 16 (4): 267–73
de Bont A, Zandwijken G, Stolk E, et al. Prioritisation by physicians in the Netherlands: the growth hormone example in drug reimbursement decisions. Health Policy 2007; 80 (3): 369–77
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. The Netherlands: reimbursement process. Lawrenceville (NJ): ISPOR, 2007 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Netherlands.asp [Accessed 2011 Mar 13]
Postma TJ, Alers JC, Terpstra S, et al. Medical technology decisions in the Netherlands: how to solve the dilemma of technology foresight versus market research? Technol Forecast Soc Change 2007; 74: 1823–33
Schafer W, Kroneman M, Boerma W, et al. The Netherlands: health system review [Health Systems in Transition]. Copenhagen: European Observatory on Health Care Systems, WHO Regional Office for Europe, 2010 [online]. Available from URL: http://www.euro.who.int/en/home/projects/observatory/publications/health-system-profiles-hits/full-list-of-hits/netherlands-hit-2010 [Accessed 2011 Mar 13]
Akkerman AE, Kuyvenhoven MM, Verheij TJ, et al. Antibiotics in Dutch general practice: nationwide electronic GP database and national reimbursement rates. Pharmacoepidemiol Drug Saf 2008; 17 (4): 378–83
de Wolf P, Brouwer WB, Rutten FF. Regulating the Dutch pharmaceutical market: improving efficiency or controlling costs? Int J Health Plann Manage 2005; 20 (4): 351–74
van Nooten F, van Agthoven M. Mandatory pharmacoeconomic studies in the Dutch reimbursement setting. Institute for Medical Technology Assessment (iMTA) Newsletter 2005; 3 (1 May): 1–3 [online]. Available from URL: http://www.imta.nl/publications/imta_newsletter_3_1.pdf [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. United States: health policy decision process. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/htaroadmaps/USHP.asp [Accessed 2011 Mar 13]
Borowski HZ, Brehaut J, Hailey D. Linking evidence from health technology assessments to policy and decision making: the Alberta model. Int J Technol Assess Health Care 2007; 23 (2): 155–61
AHTDP information and reports. Health Technologies and Services Policy Unit. Edmonton (AB): Alberta Health and Wellness, 2010 [online]. Available from URL: http://www.health.alberta.ca/initiatives/AHTDP-info-reports.html#newsletter [Accessed 2011 Mar 13]
Levin L, Goeree R, Sikich N, et al. Establishing a comprehensive continuum from an evidentiary base to policy development for health technologies: the Ontario experience. Int J Technol Assess Health Care 2007; 23 (3): 299–309
OHTAC recommendations. Decision-making framework. Toronto (ON): Ontario Health Technology Advisory Committee, 2010 [online]. Available from URL: http://www.health.gov.on.ca/english/providers/program/ohtac/decision_frame.html [Accessed 2011 Mar 13]
Ontario Health Technology Advisory Committee: evidence-based advice on technology to advance health. Toronto (ON): Ontario Health Technology Advisory Committee (OHTAC), 2010 [online]. Available from URL: http://www.health.gov.on.ca/english/providers/program/ohtac/ohtac_mn.html [Accessed 2011 Mar 13]
Medical Technology Assessment Program [MedTAP]. Salem (OR): Oregon Health Resources Commission (HRC), 2009 [online]. Available from URL: http://www.oregon.gov/OHPPR/HRC/docs/HRC.Reports/MEDTAP_09_accepted.pdf [Accessed 2011 Mar 13]
Medical Technology Assessment Program [MedTAP]. Salem (OR): Oregon Health Resources Commission (HRC), 2006 [online]. Available from URL: http://www.oregon.gov/OHPPR/HRC/docs/Policy/MedTapPolicy.pdf [Accessed 2011 Mar 13]
Health technology selection process. Olympia (WA): Washington State Health Care Authority, 2007 [online]. Available from URL: http://www.hta.hca.wa.gov/ [Accessed 2011 Mar 13]
Health technology assessment prioritization criteria. Olympia (WA): Washington State Health Care Authority, 2007 [online]. Available from URL: http://www.hta.hca.wa.gov/documents/prioritization_criteria.pdf [Accessed 2011 Mar 13]
Washington Health Technology Assessment Program (overview). Olympia (WA): Washington State Health Care Authority, 2007 [online]. Available from URL: http://www.hta.hca.wa.gov/documents/hta_overview.pdf [Accessed 2011 Mar 13]
Health technology assessment: program review. Olympia (WA): Washington State Health Care Authority, 2008 [online]. Available from URL: http://www.hta.hca.wa.gov/documents/program_review.pdf [Accessed 2011 Mar 13]
Poulin P. Developing criteria for evaluating the introduction of health technology at the local level, 2009 [online]. Available from URL: http://www.f2fe.com/CAHSPR/2009/docs/D6/d6c%20Paule%20Poulin.pdf [Accessed 2011 Mar 13]
Rochaix L, Xerri B. National Authority for Health: France. Issue Brief (Commonw Fund) 2009; 58: 1–9
Massol J, Puech A, Boissel J-P, et al. How to anticipate the assessment of the public health benefit of new medicines? Therapie 2007; 62 (5): 417–35
Chicoye A, Chhabra A. ISPOR global health care systems road map. France: pharmaceuticals. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/htaroadmaps/France.asp [Accessed 2011 Mar 13]
Chicoye A, Levesque K. ISPOR global health care systems road map. France: medical devices. Lawrenceville (NJ): ISPOR, 2010 [online]. Available from URL: http://www.ispor.org/htaroadmaps/FranceMD.asp [Accessed 2011 Mar 13]
Rapid assessment method for assessing medical and surgical procedures. Paris: Haute Autorite de Sante (HAS), Department of Medical and Surgical Procedures Assessment, 2007 [online]. Available from URL: http://www.has-sante.fr/portail/upload/docs/application/pdf/rapid_assessment_method_eval_actes.pdf [Accessed 2011 Mar 13]
Roche T, Brunet V. Medical devices evaluation by the HAS in order to be reimbursed in France. Lyon: Roche et Associés, 2009
Nguyen-Kim L, Or Z, Paris V, et al. The politics of drug reimbursement in England, France and Germany. Health Economics Letter 2005 Oct; 99: 1–6
General method for assessing health technologies. Paris: Haute Autorite de Sante (HAS), Department of Medical and Surgical Procedures Assessment, 2007 [online]. Available from URL: http://www.has-sante.fr/portail/upload/docs/application/pdf/general_method_eval_techno.pdf [Accessed 2011 Mar 13]
Orvain J, Xerri B, Matillon Y. Overview of health technology assessment in France. Int J Technol Assess Health Care 2004; 20 (1): 25–34
Haute Autorite de Sante (HAS). Paris: HAS, 2010 [online]. Available from URL: http://www.has-sante.fr [Accessed 2011 Mar 13]
French HAS recommends cutting off reimbursement for 145 more medicines. APM Health Europe, 2006 Oct 16 [online]. Available from URL: http://www.apmhe.com/story.php?mots=HAS&searchScope=1&searchType=0&depsPage=4&numero=L4207 [Accessed 2011 Mar 13]
Nasser M, Sawicki P. Institute for Quality and Efficiency in Health Care: Germany. Issue Brief (Commonw Fund) 2009; 57: 1–12
Grocott R, Metcalfe S. Going against the flow: the impact of PHARMAC not funding COX-2 inhibitors for chronic arthritis. N Z Med J 2005 Oct 7; 118 (1223): U1690
Holtorf AP, Matuszewski K, Nuijten M, et al. ISPOR global health care systems road map. Germany: pharmaceutical. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/htaroadmaps/Germany.asp [Accessed 2011 Mar 13]
The German Agency for Health Technology Assessment of DIMDI. Cologne: German Institute of Medical Documentation and Information (DIMDI), 2009 [online]. Available from URL: http://www.dimdi.de/static/en/hta/dahta/index.htm [Accessed 2011 Mar 13]
Dintsios GA. The impact of HTA reports on decision-making processes in the health sector in Germany: executive summary [HTA Report Executive Summary]. Cologne: German Agency for Health Technology Assessment at the German Institute of Medical Documentation and Information (DIMDI), 2006 [online]. Available from URL: http://portal.dimdi.de/de/hta/hta_berichte/hta031_summary_en.pdf [Accessed 2011 Mar 13]
Schiffner R. Criteria used by the KBV-innovation service for decision on proposals of medical, non-pharmaceutical innovations to the German Federal Joint Committee (G-BA) [abstract]. Edmonton (AB): Health Technology Assessment International (HTAi), 2007
Pharmaceutical pricing and reimbursement information: Germany. Vienna: Pharmaceutical Pricing and Reimbursement Information-PPRI, 2008 [online]. Available from URL: http://ppri.oebig.at/Downloads/Results/Germany_PPRI_2008.pdf [Accessed 2011 Mar 13]
Gress S, Niebuhr D, May W, et al. Reform of prescription drug reimbursement and pricing in the German social health insurance market: a comparison of three scenarios. Pharmacoeconomics 2007; 25 (6): 443–54
The Federal Joint Committee: about us. Berlin: Gemeinsamer Bundesausschuss, Institut fur Qualitat und Wirtschaftlichkeit in Gesundheitswesen, 2010 [online]. Available from URL: http://www.g-ba.de/downloads/17-98-2804/2010-01-01-Faltblatt-GBA_engl.pdf [Accessed 2011 Mar 13]
Institute for Quality and Efficiency in Health Care. General methods for the assessment of the relation of benefits to costs. Version 1.0. Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009 [online]. Available from URL: http://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf [Accessed 2011 Mar 13]
Institute for Quality and Efficiency in Health Care: general methods. Cologne: Institute for Quality and Efficiency in Health Care, 2008 [online]. Available from URL: http://www.iqwig.net/methods-procedures.926.en.html [Accessed 2011 Mar 13]
Coughlan JJ, Fortescue-Webb D, Heaney R, et al. ISPOR global health care systems road map. Ireland: pharmaceutical. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Ireland.asp [Accessed 2011 Mar 13]
Usher C, Tilson L, Barry M. Evidence of the positive impact of health technology assessment on healthcare decision-making in Ireland [abstract]. Edmonton (AB): Health Technology Assessment International (HTAi), 2008: 607
Tilson L, O’Leary A, Usher C, et al. Pharmacoeconomic evaluation in Ireland: a review of the process. Pharmacoeconomics 2010; 28 (4): 307–22
Barry M, Tilson L. Recent developments in pricing and reimbursement of medicines in Ireland. Expert Rev Pharmacoecon Outcomes Res 2007; 7 (6): 605–11
Persson U, Willis M, Odegaard K. A case study of ex ante, value-based price and reimbursement decision-making: TLV and rimonabant in Sweden. Eur J Health Econ 2010 Apr; 11 (2): 195–203
Nygren P, Sandman L. If you are young you get it, but if you are old you are left out: the significance of age for choice of treatment and priorities in cancer care. Lakartidningen 2008; 105 (47): 3417–9
Jansson S. Implementing accountability for reasonableness: the case of pharmaceutical reimbursement in Sweden. Health Econ Policy Law 2007; 2 (Pt 2): 153–71
Faulkner E, Matuszewski K, Niziol C. ISPOR global health care systems road map. Sweden: pharmaceutical. Lawrenceville (NJ): ISPOR, 2009 [online]. Available from URL: http://www.ispor.org/HTARoadMaps/Sweden.asp [Accessed 2011 Mar 13]
Anell A, Persson U. Reimbursement and clinical guidance for pharmaceuticals in Sweden: do health-economic evaluations support decision making? Eur J Health Econ 2005; 6 (3): 274–9
Working guidelines for the pharmaceutical reimbursement review. Stockholm: Tandvards-Och Lakemedels-formansverket (TLV)/the Dental and Pharmaceutical Benefits Agency [formerly the Swedish Pharmaceutical Benefits Board], 2008 [online]. Available from URL: http://www.tlv.se/Upload/Genomgangen/guidelines-pharmaceutical-reimbursement.pdf [Accessed 2011 Mar 13]
Welcome to TLV [the Dental and Pharmaceutical Benefits Agency]. Stockholm: Tandvards-Och Lakemedelsfor-mansverket (TLV)/the Dental and Pharmaceutical Benefits Agency, 2008 [online]. Available from URL: http://www.tlv.se/in-english/ [Accessed 2011 Mar 13]
Reimbursement review. Stockholm: Tandvards-Och Lakemedelsformansverket (TLV)/the Dental and Pharmaceutical Benefits Agency, 2008 [online]. Available from URL: http://www.tlv.se/in-english/reimbursement-review/[Accessed 2011 Mar 13]
Guidelines for companies: the Swedish Pharmaceutical Benefits Board (LFN). Stockholm: Lakemedels-formansnamnden (LFN)/the Swedish Pharmaceutical Benefits Board, 2008 [online]. Available from URL: http://www.tlv.se/Upload/English/Guidelines-for-Companies.pdf [Accessed 2011 Mar 13]
Pharmaceutical pricing and reimbursement information: Sweden. Vienna: PPRI-Pharmaceutical Pricing and Reimbursement Information, 2007 [online]. Available from URL: http://ppri.oebig.at/Downloads/Results/Sweden_PPRI_2007.pdf [Accessed 2011 Mar 13]
Chalkidou K. Comparative effectiveness review within the UK’s National Institute for Health and Clinical Excellence. Issue Brief (Commonw Fund) 2009; 59: 1–12
Karnon J, Carlton J, Czoski-Murray C, et al. Informing disinvestment through cost-effectiveness modelling: is lack of data a surmountable barrier? Appl Health Econ Health Policy 2009; 7 (1): 1–9
Mason AR, Drummond MF. Public funding of new cancer drugs: is NICE getting nastier? Eur J Cancer 2009; 45 (7): 1188–92
Parrish A, Blockman M. Clinical excellence and the NIC-Eties of value-based priority setting. S Afr Med J 2008; 98 (10): 758, 760–1
Syrett K. NICE and judicial review: enforcing ‘accountability for reasonableness’ through the courts? Med Law Rev 2008; 16 (1): 127–40
Williams IP, Bryan S. Cost-effectiveness analysis and formulary decision making in England: findings from research. Soc Sci Med 2007; 65 (10): 2116–29
Summerhayes M, Catchpole P. Has NICE been nice to cancer? Eur J Cancer 2006; 42 (17): 2881–6
Supporting rational local decision-making about medicines (and treatments): a handbook of good practice guidance. Executive summary. 1st ed. Liverpool: National Prescribing Centre, 2009 [online]. Available from URL: http://www.npc.co.uk/policy/resources/handbook_executive.pdf [Accessed 2011 Mar 13]
Useful sources of information for area prescribing and medicines management committees (APCs). Liverpool: National Prescribing Centre, 2009 [online]. Available from URL: http://www.npc.co.uk/policy/resources/apc_guide_resources.pdf [Accessed 2011 Mar 13]
Defining guiding principles for processes supporting local decision making about medicines. Final report. Liverpool: National Prescribing Centre, 2009 [online]. Available from URL: http://www.midessex.nhs.uk/Documents/Key-Documents/Policies%20and%20Procedures/Medicines%20Management%20Policies%20and%20Procedures/DH%20Defining%20Guiding%20Principles%20for%20Processes%20supporting%20Local%20Decision%20Making%20about%20Medicines%20Report.pdf [Accessed 2011 Mar 13]
Reeve S. Directions to primary care trusts and NHS trusts concerning decisions about drugs and other treatments 2009. London: Department of Health, 2009 [online]. Available from URL: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsLegislation/DH_096067 [Accessed 2011 Mar 13]
Brambleby P, Jackson A, Muir Gray JA. Programme-based decision-making for better value healthcare: second annual population value review. Oxford (UK): NHS National Knowledge Service, 2008 [online]. Available from URL: http://www.nks.nhs.uk/APVR2.pdf [Accessed 2011 Mar 13]
Wilson G. ISPOR global health care systems road map. United Kingdom: diagnostics. Lawrenceville (NJ): ISPOR, 2010 [online]. Available from URL: http://www.ispor.org/htaroadmaps/UKDiagnostics.asp [Accessed 2011 Mar 13]
International Society for Pharmacoeconomics and Outcomes Research. ISPOR global health care systems road map. United Kingdom (England and Wales): reimbursement process. Lawrenceville (NJ): ISPOR, 2008 [online]. Available from URL: http://www.ispor.org/htaroadmaps/UK.asp [Accessed 2011 Mar 13]
Single technology appraisal (STA): specification for manufacturer/sponsor submission of evidence. London: NICE, 2009 [online]. Available from URL: http://www.nice.org.uk/media/59C/B3/SpecificationForManufacturerSponsorSubmissionEvidenceJune2010.doc [Accessed 2011 Mar 13]
National Institute for Health and Clinical Excellence. Guide to the single technology appraisal (STA) process. London: NICE, 2009 [online]. Available from URL: http://www.nice.org.uk/media/913/06/Guide_to_the_STA-proof_6-26-10-09.pdf [Accessed 2011 Mar 13]
Guide to the multiple technology appraisal process. London: NICE, 2009 Oct [online]. Available from URL: http://www.nice.org.uk/media/916/6B/Guide_to_the_MTA-proof_8-26-10-09.pdf [Accessed 2011 Mar 13]
McCabe C, Chilcott J, Claxton K, et al. Continuing the multiple sclerosis risk sharing scheme is unjustified. BMJ 2010 Jun 3; 340:c1786
Neumann PJ, Divi N, Beinfeld MT, et al. Medicare’s national coverage decisions, 1999–2003: quality of evidence and review times. Health Aff (Millwood) 2005; 24 (1): 243–54
Tunis S, Whicher D. The National Oncologic PET Registry: lessons learned for coverage with evidence development. J Am Coll Radiol 2009; 6 (5): 360–5
Neumann PJ, Tunis SR. Medicare and medical technology: the growing demand for relevant outcomes. N Engl J Med 2010; 362 (5): 377–9
The Lewin Group, Inc. Cost-effectiveness considerations in the approval and adoption of new health technologies: final report and case studies. Washington, DC: Department of Health and Human Services, Assistant Secretary for Planning and Evaluation, 2007 [online]. Available from URL: http://aspe.hhs.gov/sp/reports/2007/cecht/index.htm [Accessed 2011 Mar 13]
Jennings ET, Hall JL. Evidence-based practice and the use of information in state agency decision-making. IFIR working paper no. 2009–10. Lexington (KT): University of Kentucky, Institute for Federalism and Intergovernmental Relations, 2009 [online]. Available from URL: http://www.ifigr.org/workshop/spring09/jennings.pdf [Accessed 2011 Mar 13]
Barnett PG. How can cost effectiveness analysis be made more relevant to US health care? Health Economics Resource Center, 2009 [online]. Available from URL: http://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/hcea-052709.pdf [Accessed 2011 Mar 13]
Factors CMS considers in commissioning external technology assessments: guidance for the public, industry, and CMS staff. Baltimore (MD): US Department of Health and Human Services, Centers for Medicare and Medicaid Services (CMS), 2006 [online]. Available from URL: https://www.cms.gov/medicare-coverage-database/details/medicare-coverage-document-details.aspx?MCDId=7&McdName=Factors+CMS+Considers+in+Commissioning+External+Technology+Assessments&mcdtypename=Guidance+Documents&MCDIndexType=1&bc=BAAIAAAAAAAA& [Accessed 2011 Mar 13]
Factors CMS considers in opening a National Coverage Determination: guidance for the public, industry, and CMS staff. Baltimore (MD): US Department of Health and Human Services, Centers for Medicare and Medicaid Services (CMS), 2006 [online]. Available from URL: https://www.cms.gov/medicare-coverage-database/details/medicare-coverage-document-details.aspx?MCDId=6&McdName=Factors+CMS+Considers+in+Opening+a+National+Coverage+Determination&mcdtypename=Guidance+Documents&MCDIndexType=1&bc=BAAIAAAAAAAA& [Accessed 2011 Mar 13]
National Coverage Determinations with data collection as a condition of coverage: coverage with evidence development. Guidance for the public, industry, and CMS staff. Baltimore (MD): US Department of Health and Human Services, Centers for Medicare and Medicaid Services (CMS), 2006 [online]. Available from URL: https://www.cms.gov/medicare-coverage-database/details/medicare-coverage-document-details.aspx?MCDId=8&McdName=National+Coverage+Determinations+with+Data+Collection+as+a+Condition+of+Coverage%3A+Coverage+with+Evidence+Development&mcdtypename=Guidance+Documents&MCDIndexType=1&bc=BAAIAAAAAAAA& [Accessed 2011 Mar 13]
Factors CMS considers in referring topics to the Medicare Evidence Development and Coverage Advisory Committee: guidance for the public, industry, and CMS staff. Baltimore (MD): US Department of Health and Human Services. Centers for Medicare and Medicaid Services (CMS), 2006 [online]. Available from URL: https://www.cms.gov/medicare-coverage-database/details/medicare-coverage-document-details.aspx?MCDId=10&McdName=Factors+CMS+Considers+in+Referring+Topics+to+the+Medicare+Evidence+Development+%26+Coverage+Advisory+Committee&mcdtypename=Guidance+Documents&MCDIndexType=1&bc=BAAIAAAAAAAA&?fromdb=true [Accessed 2011 Mar 13]
Guiding principles for when National Coverage Determination topics are referred for external expertise via a technology assessment and/or the Medicare Coverage Advisory Committee. Draft guidance — not for implementation. Baltimore (MD): US Department of Health and Human Services, Centers for Medicare and Medicaid Services (CMS)], 2003 [online]. Available from URL: https://www.cms.gov/FACA/Downloads/guidelines.pdf [Accessed 2011 Mar 13]
Stafinski T, McCabe CJ, Menon D. Funding the unfundable: mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. Pharmacoeconomics 2010; 28 (2):113–42
National Institute for Health and Clinical Excellence. First report of session 2007–08. Volume 1. Report, together with formal minutes. London: House of Commons. Health Committee, 2008 [online]. Available from URL: http://www.publications.parliament.uk/pa/cm200708/cmselect/cmhealth/27/27.pdf [Accessed 2011 Mar 13]
Review of health technology assessment in Australia: discussion paper 4. Improved administration of commonwealth HTA processes. Canberra (ACT): Department of Health and Ageing, 2009 [online]. Available from URL: http://www.health.gov.au/internet/main/publishing.nsf/Content/9CB872326EA192E5CA25764100024D0C/$File/discussionpaper4.pdf [Accessed 2011 Mar 13]
Skinner BJ, Rovere M. Access delayed, access denied: waiting for new medicines in Canada. 2010 report [Studies in Pharmaceutical Policy]. Vancouver (BC): Fraser Institute, 2009 [online]. Available from URL: http://www.fraseramerica.org/commerce.web/product_files/accessdelayedaccessdenied2010_US.pdf [Accessed 2011 Mar 13]
Gafni A, Birch S. Incremental cost-effectiveness ratios (ICERs): the silence of the lambda. Soc Sci Med 2006 May; 62 (9): 2091–100
Birch S, Gafni A. The biggest bang for the buck or bigger bucks for the bang: the fallacy of the cost-effectiveness threshold. J Health Serv Res Policy 2006 Jan; 11 (1): 46–51
Busse R. Priority-setting and rationing in German health care. Health Policy 1999; 50 (1–2): 71–90
Kennedy I. Appraising the value of innovation and other benefits: a short study for NICE. London: NICE, 2009 [online]. Available from URL: http://www.nice.org.uk/media/98F/5C/KennedyStudyFinalReport.pdf [Accessed 2011 Mar 13]
Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004; 329 (7459): 224–7
Davies C, Wetherell M, Barnett E, et al. Opening the box: evaluating the Citizens Council of NICE. Buckingham: Open University Press, 2005 [online]. Available from URL: http://www.pcpoh.bham.ac.uk/publichealth/methodology/docs/invitations/Citizens_council_Mar05.pdf [Accessed 2011 Mar 13]
Report of the Provincial Working Group on the Delivery of Oncology Medications for Private Payment in Ontario Hospitals. Toronto (ON): Council of Academic Teaching Hospitals of Ontario (CAHO), Ontario Hospital Association (OHA), Cancer Care Ontario (CCO), Ontario Ministry of Health and Long-Term Care, 2006 Jul 27 [online]. Available from URL: http://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13638 [Accessed 2011 Mar 13]
Glen C, Skinner B. The common drug review: governments avoiding accountability for rationing. Fraser Forum 2006 June: 16–9 [online]. Available from URL: http://www.fraserinstitute.org/uploadedFiles/fraser-ca/content/research-news/research/articles/TheCommonDrugReview.pdf [Accessed 2011 Mar 13]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stafinski, T., Menon, D., Philippon, D.J. et al. Health Technology Funding Decision-Making Processes Around the World. Pharmacoeconomics 29, 475–495 (2011). https://doi.org/10.2165/11586420-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11586420-000000000-00000