Abstract
The recent approval of human papillomavirus (HPV) vaccine means that decision makers need information beyond that available from randomized clinical trials to recommend funding for this vaccination programme. Modelling and economic studies have addressed some of those information needs. We conducted a qualitative systematic review to summarize the existing data. Review articles were obtained from an extensive literature search on studies using mathematical modelling (either a Markov or transmission dynamic model) to determine the effectiveness or cost effectiveness of an HPV vaccine compared with the current cytology-based Pap smear screening programme.
A total of 21 studies (but 22 models) were included in the review after being assessed for methodological quality. All of the included studies had used a mathematical model to determine the effectiveness of an HPV vaccine, whilst 13 had also conducted a cost-effectiveness analysis. Although the studies used different model structures, baseline parameters and assumptions, all studies showed that vaccination would decrease rates of HPV infection, precancerous lesions and cervical cancer. Studies had a consistent message with respect to cost effectiveness: a female-only vaccination programme is cost effective compared with the current cytology-based Pap smear screening programme, while the cost effectiveness of a male and female vaccination programme is generally not cost effective compared with female-only vaccination.
Similar content being viewed by others

Human papillomavirus (HPV) infection can cause cervical, anogenital and head and neck cancers as well as genital warts and recurrent respiratory papillomatosis.[1–7] However, the greatest impact of HPV disease relates to cervical cancer, which is the second most common cancer in women worldwide.[8] Every year, approximately 500 000 women are diagnosed with cervical cancer, and approximately 300 000 die from the disease.[8,9]
About 70% of cervical cancer disease is caused by HPV-16 and -18.[10] Recently, two vaccines designed to prevent HPV infection have been introduced into the world markets: a quadrivalent recombinant vaccine (HPV-6, -11, -16, -18), Gardasil™ (Merck Frosst Ltd, Montreal, QC, Canada) and a bivalent vaccine (HPV-16, -18), Cervarix™ (GlaxoSmithKline, Toronto, ON, Canada).[11,12] Clinical trials of these vaccines have shown a high degree of efficacy in the prevention of infections and precancerous cervical intraepithelial neoplasia (CIN) lesions related to HPV-16 and -18.[13–19]
Ideally, the clinical trials would have used prevention of cervical cancer as their efficacy endpoint.[20] However, this was not feasible with the HPV vaccine trials, as the cancer typically develops 20 years after HPV infection.[20] In addition, the standard of care in developed countries is to screen for precancerous lesions via Pap smear screening programmes and to excise CIN grade 2 or 3 lesions before the development of cancer.[20] Thus, several investigators[21,22] have used mathematical modelling to project the impact of vaccination programmes on cervical cancer rates, and to determine the long-term benefits of vaccination. In addition, some of these models have incorporated economic parameters to determine the most cost-effective strategy.[23,24]
Our objective was to conduct a systematic review of the studies that used modelling to determine the potential effectiveness and cost effectiveness of an HPV vaccination programme.
1. Literature Review
1.1 Search Strategy
We conducted a systematic review of the literature from 1966 to 2008 by searching MEDLINE (Ovid system), EMBASE, Cochrane Collaboration of Systematic Reviews, Health Technology Assessment and Centre for Review and Dissemination for articles and reviews pertaining to cost effectiveness of the HPV vaccine. The following medical subject heading terms were used for this review: ‘human papillomavirus’, ‘HPV’, ‘vaccine’, ‘vaccination’, ‘cost’, ‘cost-effectiveness’, ‘economic evaluation’, ‘mathematical models’, ‘modeling’ and ‘pharmacoeconomics’. A grey literature search was conducted using the following links to identify published and unpublished articles and abstracts: PapersFirst, Proceeding first, Google scholar and governmental agencies such as the Canadian Agency for Drug and Technologies in Health, UK National Institute for Health and Clinical Excellence (NICE) and US FDA.
1.2 Inclusion Criteria and Quality Assurance
Studies included in this review were English-language articles that evaluated the effectiveness or cost effectiveness of HPV vaccination compared with the current cytology-based Pap smear screening programme.
All the articles were assessed for methodological quality by two reviewers (B. Oteng and K. Cloutier) using the following criteria: viewpoint, provision of full economic comparison, use of appropriate intervention, proper measurement of valuation and outcome, allowance for uncertainty analysis, adequate information on source of resource used, adequate information on effectiveness data and unit cost used and use of reasonable model structure and parameters, as outlined in a user guide by Drummond et al.[25] If the two reviewers did not agree, then the primary investigator (F. Marra) also reviewed the study.
1.3 Data Extraction
The following information was obtained from each study included in this systematic review: model type; perspective; time horizon; target HPV types; age of vaccination; assumptions on coverage; uptake; requirement of a booster dose; efficacy; duration of protection; costs; reduction in cervical cancer-related mortality; reduction in cervical cancer cases; reduction in pre-cancer lesions CIN-1, -2, -3; reduction in HPV infection; reduction in genital warts; incremental cost associated with vaccination programme; incremental life-years (LYs) or QALYs gained from a vaccination programme.
1.4 Type of Mathematical Models
Two types of mathematical models have been used to evaluate the long-term effectiveness of a vaccination programme: static Markov and transmission dynamic models.[23,24]
A static Markov model (also referred to as a cohort model) uses probabilistic event simulation for modelling of short- and long-term processes. In this type of model, each individual can reside in only one health state at any point in time and transitions occur from one health state to the other at defined intervals of equal length according to transition probabilities based on population characteristics (age, sexual risk, HPV type). The process can be repeated until all the cohort has advanced to the death compartment and then survival time and healthcare costs are calculated with the time spent in each compartment over the lifetime of the cohort. For HPV, the different states are usually susceptible (have not been infected), infected, CIN and invasive cervical cancer (ICC). Some models may also include screening and treatment compartments that modify the transition probabilities.
On the other hand, a dynamic model can be deterministic and nonlinear (unlike a Markov model). Instead of following a single cohort, it tracks a changing population over time. In a dynamic model, individuals constantly enter the model as they are born and exit it as they die, which means that, as long as people are being born, the model does not have a natural stopping point. In this type of model, individuals are susceptible, infected or immune (immunized individuals who have recovered from an infection or have been vaccinated), or progress on to CIN and ICC.
The main difference between Markov and dynamic modelling is that the latter accounts for the HPV vaccination reducing the prevalence of infection in the population over time. Thus, dynamic modelling has the advantage of properly assessing the impact of herd immunity (which is a decreased prevalence of infection over time because of fewer infected individuals in the population that are likely to infect others). Incorporating the contribution of herd immunity in pharmacoeconomic analyses is less likely to lead to underestimation of the cost effectiveness of the evaluated programme. Another advantage of dynamic modelling is that it allows more flexibility in capturing multiple dimensions of heterogeneity (such as type-specific HPV and individual-based risk factors), permits the risk of future events to depend on one or more prior events and allows evaluation of a female-only versus a male-female vaccination programme. Limitations of dynamic models include that, in addition to reflecting disease natural history, they must also capture mixing patterns between risk classes to accurately reflect transmission dynamics — these data are particularly sparse. In addition, dynamic transmission models require more model parameters, which tends to increase the level of model uncertainty.
1.5 Search Results
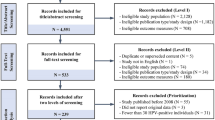
The literature search found 128 studies, 25 of which were duplicates and 82 of which did not meet study criteria and so were excluded (figure 1). A total of 21 articles were included for the systematic analysis;[26–31,33–41,43–45,49,51,52] however, Hughes et al.[26] reported their results using two model types, and thus, 22 models are described in our analysis. Ten of these are Markov models,[26–31,33–36] one group of investigators used a hybrid model (combining a dynamic and Markov model)[37] and 11 were dynamic models.[26,38–41,43–45,49,51,52] Seven[27,28,30,33–36] of the ten Markov models, the one hybrid model,[37] and 5 of the 11 dynamic studies[40,43,45,49,52] focused on both modelling effectiveness and cost-effectiveness analysis, with the remainder focusing only on modelling effectiveness. All studies projected the impact of an HPV vaccination programme on HPV prevalence, CIN and cervical cancer incidence, but only some studies included decreased incidence of genital warts as an outcome.[33,35,40,43]
2. Modelling Effectiveness
2.1 Markov Models
2.1.1 Hughes et al., 2002
Hughes et al.[26] created a transition-state Markov model looking at the effect of vaccinating a cohort of 16-year-old females against 60% of high-risk HPV types on the incidence of carcinoma in situ (CIS) and ICC. The cohort was divided into four classes of sexual activity and was followed until all individuals were dead (by age 75 years). In this model, they looked at four different states: susceptible, HPV infected, cervical cancer and death. The authors did not include a CIN state but did look at the impact of screening on the incidence of CIS. With these parameters, they estimated that HPV vaccination would reduce CIS by 46% and cervical cancer by 47%. The magnitude of the decrease was similar in both the screened and unscreened populations and the authors suggested that this was due to the potential replacement effect, in which individuals who would have developed cancer because of HPV-16, -18 would now develop it because of other high-risk HPV types.
2.1.2 Sanders and Taira, 2003
Sanders and Taira[27] used a Markov model to evaluate the effectiveness and cost effectiveness of a prophylactic vaccine against 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). Their model simulated the progression of HPV in a hypothetical cohort of vaccinated and non-vaccinated 12-year-old girls over their lifetimes. The authors used a model previously developed by Myers et al.[58] to simulate both high- and low-risk HPV types. Their transition model comprised five different states as opposed to compartments: (i) susceptible; (ii) HPV infected; (iii) squamous intraepithelial lesions (SIL); (iv) cervical cancer; and (v) death. The authors based their probability estimates for HPV infection, SIL, cervical cancer and death on large, high-quality studies reported in the literature. They assumed a school-based vaccination programme with 70% vaccine coverage, 75% efficacy and a 10-year duration of protection (booster given every 10 years). They also assumed that 71% of the cohort would receive standard care (defined as Pap testing every 2 years beginning at the age of 16 years). Their model showed a reduction in incidence of HPV infection of 13.3% and a reduction in SIL of 21.2% with vaccination plus standard care versus standard care alone. Cancer incidence was also reduced by 19.9% and cancer-related death by 20.7%.
2.1.3 Kulasingam and Myers, 2003
Kulasingam and Myers[28] examined the potential health and economic effects of an HPV vaccine targeted at 70% of HPV high-risk types (including HPV-16, -18), in the setting of existing cervical cancer screening, using a state-transition Markov model. They used relevant parameters from a published systematic review for the natural history of HPV infection and cervical cancer. The investigators simulated both high- and low-risk HPV types. They followed a cohort of 12-year-old girls until the age of 85 years and evaluated three different strategies: vaccination without screening, screening without vaccination, and vaccination plus screening. There were seven states in their transition model: (i) susceptible; (ii) low-risk HPV infection; (iii) high-risk HPV infection; (iv) CIN-1; (v) CIN-2, -3; (vi) four cervical cancer states according to the International Federation of Gynaecology and Obstetrics (stages I–IV); and (vii) death. They examined the effect of starting screening at different ages (18–22, 24, 26 and 30 years) and various screening intervals (every 1, 2, 3 and 5 years). The authors assumed 100% vaccine coverage and 90% effectiveness, with a 10-year duration of protection (no information regarding booster shot requirement was included in the study protocol). Based on these parameters, the authors reported that the model predicted a lowered prevalence of high-risk HPV when a longer duration of vaccine efficacy was assumed. For a strategy including biennial screening starting at the age of 18 years plus vaccination there was a reduction in cervical cancer incidence of 15% compared with screening alone.
2.1.4 Goldie et al., 2003
Goldie et al.[29] examined the impact of a prophylactic HPV vaccine against high-risk types HPV-16, -18 on the risk of ICC, precursor cervical lesions and type-specific infection with HPV. They followed a cohort of 100 000 vaccinated and non-vaccinated girls aged 13 years through the natural history of HPV disease for their lifetimes. Their model was divided into nine transition states: (i) susceptible; (ii) persistent HPV-16, -18 infection; (iii) persistent non-HPV-16, -18 infection; (iv) persistent low-risk HPV types of infection; (v) transient low- or high-risk types of HPV infection; (vi) CIN-1; (vii) CIN-2, -3; (viii) four categories of ICC based on the staging of the International Federation of Gynaecology and Obstetrics (stages I–IV); and (ix) death. The authors assumed 100% vaccine coverage, various vaccine efficacies (50%, 75% and 98%) and lifelong immunity for 12-year-old girls. From these parameters, Goldie et al.[29] estimated that a vaccine with 98% efficacy against HPV-16, -18 was associated with an equivalent reduction in persistent cancer associated with the same HPV types and a 51% reduction in total cervical cancer incidence. With a decrease in vaccine effectiveness to 75%, the reductions in HPV-16, -18-associated cancer and total cervical cancer were 85% and 44%, respectively.
2.1.5 Goldie et al., 2004
The following year, Goldie et al.[30] published a second study looking at the clinical benefits and cost effectiveness of a bivalent HPV vaccine in the setting of an existing Pap smear screening programme. They used the same transition states as in their previous study for a hypothetical cohort of 100 000 girls aged 13 years (vaccinated at 12 years, before sexual debut) and assumed 100% vaccine coverage, 90% efficacy and lifelong immunity. They also assumed that 71% of women would be screened every year. Based on these assumptions, the authors suggested that adding a vaccination programme to current screening would reduce the age-specific prevalence of low-grade SIL (LSIL) and high-grade SIL (HSIL). They achieved a greater reduction in the incidence of HSIL because the proportion of HSIL attributable to HPV-16, -18 is greater than the proportion of LSIL associated with these two high-risk types (specific reduction rates not stated in the article). The reduction in lifetime risk of cervical cancer was reduced by 58.1% with the current US screening programme plus vaccination compared with screening alone. When vaccine effectiveness was 70%, the reduction in cancer risk was 45.3%, and with 100% effectiveness, it was 65.1%. When screening was initiated at 25 years and performed every 5 years, the model showed that adding HPV vaccination decreased cervical cancer risk by 60.9% (when conventional cytology was used), whereas it was reduced by 61.2% when initiated at 30 years and performed at the same intervals.
2.1.6 Kohli et al., 2007
Kohli et al.[31] published a generic transition-state Markov model that could be used to evaluate the impact of an HPV-16, -18 vaccine in different countries, and reported the use of the model in the UK. In their model, individuals advanced through eight states: (i) susceptible; (ii) HPV infected; (iii) CIN-1; (iv) CIN-2; (v) CIN-3; (vi) four stages of ICC according to the International Federation of Gynaecology and Obstetrics; (vii) ICC-related death; and (viii) non-ICC-related death. The ages of Pap smear test screening, coverage rates and screening practices were based on data reported by the Cervical Screening Programme from the UK Department of Health Bulletin.[32] The parameters included in the model regarding HPV natural history were based on a comprehensive review of the literature. They examined the impact of vaccination of a cohort of 12-year-old girls (n = 376 385) and made the following assumptions: 100% vaccine coverage; 95% efficacy against HPV-16, -18; cross protection offering 50% and 90% reduction in HPV-31 and -45 infections, respectively; and lifelong immunity.
Based on these assumptions, Kohli et al.[31] estimated a 95% reduction in the prevalence of lesions associated with HPV-16 and -18 with a 66.3% reduction in HSIL from all HPV types for vaccination and screening compared with screening alone. Vaccination also reduced incidence of CIN-1 lesions caused by all types of HPV by 31%. The investigators predicted that vaccination would result in a 76.0% reduction in cervical cancer incidence and a 76.1% reduction in cancer-related death. In a sensitivity analysis, the authors estimated that, without cross protection against HPV-31 and -45, the incidence of cervical cancer would be reduced by 73.1% with vaccination. They also estimated that vaccine coverage of 80% would lead to a lower reduction in cancer cases (73.1%) than with 100%. Vaccination of 10-year-old girls instead of 12-year-olds did not have an impact on cervical cancer incidence, whereas delaying vaccination to age 18 years resulted in a lower reduction rate (66.0%) in high-grade lesions.
2.1.7 Brisson et al., 2007
Brisson et al.[33] published a compartmental deterministic model that followed a cohort of 100 000 women through eight states: (i) susceptible; (ii) HPV infected; (iii) immune; (iv) genital warts; (v) CIN-1; (vi) CIN-2, -3; (vii) cervical cancer; and (viii) death. The model evaluated HPV-16, -18, low- and high-risk types and assumed lifelong immunity following infection and vaccination. The model compared the bivalent and quadrivalent vaccine versus no vaccination, under the current Pap smear screening programme. They assumed vaccination of 12-year-old girls, 95% vaccine efficacy, 100% uptake and vaccination cost of $US400 per course. Under base-case assumptions, the model predicted that the quadrivalent HPV vaccine would prevent 18 000 episodes of genital warts (86% risk reduction), 20 000 cases of CIN-1 (24% risk reduction), 13 000 cases of CIN-2, -3 (47% risk reduction), 310 cases of cervical cancer (62% risk reduction) and 140 cervical cancer-related deaths over the lifetime of the cohort. The numbers were the same for the bivalent vaccine except there were no reductions in cases of genital warts and 16 000 cases of CIN-1 (19% risk reduction) were prevented.
2.1.8 Kulasingam et al., 2007
Kulasingam et al.[34] assessed the effectiveness of the HPV vaccine using a Markov model. They simulated a cohort of females beginning at the age of 12 years until 85 years. The different health states used in their model were HPV infection, CIN-1, -2, -3 and International Federation of Gynaecology and Obstetrics Cancer stages I–IV. The data were further stratified by HPV type (low-risk HPV, non-16, -18 high-risk HPV and HPV-16, -18). Base-case assumptions were 80% vaccine coverage, 100% vaccine efficacy and lifelong duration of protection. A vaccine cost of $US115 per dose was used; however, no booster coverage was assumed. Administration of vaccine was assumed to be through school-based programmes for individuals aged ≤18 years, and through a primary care GP for those aged 18–26 years. In sensitivity analysis, the cost effectiveness of the catch-up programme offered to 14- to 26-year-olds was determined. Pre- and post-vaccination cumulative incidence of HPV was used to calculate the relative risk of HPV infection. The model predicted a 2.4% lifetime risk of cancer without screening, 0.77% with screening and 0.37% with vaccination.
2.1.9 Kulasingam et al., 2008
Kulasingam et al.[35] used a previously published and validated state-transition Markov model of HPV to estimate incremental cost-effectiveness ratios (ICERs), total lifetime costs and life expectancy associated with different screening strategies either alone or in combination with HPV vaccination. Their study targeted HPV-6, -11, -16 and -18 for the UK population. Estimates and ranges for the natural history of the HPV infection and yearly transition probabilities were obtained from other studies. Their model simulated a cohort of women aged 12 years and followed them to the age of 85 years. The different health states modelled were HPV infection, CIN-1, -2, -3, and International Federation of Gynaecology and Obstetrics stages I–IV. Furthermore, the model was calibrated to produce prevalence curves for HPV infection, lifetime risk of cervical cancer and incidence of cervical cancer.
The following base-case assumptions were used: women aged 25–49 years were screened every 3 years, women aged 50–64 years were screened every 5 years, treatment of CIN was 100% effective, 20% of women with CIN-1 were assumed to be treated; a 98% effectiveness of the quadrivalent vaccine against cervical cancer and genital warts; duration of immunity was lifetime; vaccine was administered to 12-year-old girls in the school-based programme; and 85% vaccine coverage. In addition, the study assumed that 90% of genital warts were associated with infection from HPV-6 and -11, and a reduction in incidence of approximately 35% for CIN-1, 55% for CIN-2, -3 and 70% for cervical cancer in the vaccinated group. Health outcomes and costs were discounted at 3.5% annually for base-case scenarios. When vaccination was added to screening, and assuming a cohort of 100 000 women, the model showed that the lifetime risk of cervical cancer was reduced from 0.71% to 0.29%, and that 418 cervical cancers, 127 deaths, 2554 cases of CIN-1, 1683 cases of CIN-2, 2479 cases of CIN-3 and 4798 cases of genital warts would be avoided.
2.1.10 Bergeron et al., 2008
Bergeron et al.[36] used a US model but fitted data to French local epidemiology and economic data. The Markov model they used followed a cohort of females from the age of 14 years to 85 years through different health states representing the natural history of HPV infection through to CIN ICC and to genital warts. Yearly transition probabilities were assigned for movement between the different health states. Several underlying assumptions were made about the different health states. For instance, women who developed CIN-1, -2 or -3 were at risk of cervical cancer but could return to ‘well’ states after cervical cancer lesion treatment. It was assumed that vaccination prevented 75% of cervical cancer caused by HPV-16 and -18, and 90% of genital warts cases; 80% vaccine coverage; and lifetime duration of protection. Under base-case assumptions, the model predicted a lifetime cervical cancer risk of 0.94% and a lifetime mortality risk of 0.22% for women who underwent cervical cancer screening. The introduction of a vaccination programme combined with screening decreased these risks by about 65% to 0.33% and 0.08%, respectively. Finally, based on a cohort of 370 000 French women, the model predicted that vaccination could avoid 0.6% of cervical cancer cases, 0.14% of cervical cancer deaths, 1.70%, 1.84% and 2.43% cases of CIN-1, -2, and -3, respectively, and 6.24% cases of genital warts.
2.2 Hybrid Models
2.2.1 Taira et al., 2004
Taira et al.[37] evaluated a wide range of vaccine efficacies and penetration to understand benefits and cost effectiveness of a female-only or male-female vaccination programme against HPV-16, -18. They used a dynamic modelling process to generate HPV infection rates and then incorporated these in the transition model of Sanders and Taira[27] They structured their HPV transmission model according to both age and sexual activity. They divided the population into nine age categories (12–50 years) and further divided these into four subcategories based on sexual activity. In their model, mixing between sexual activity groups was assumed to be assortive, with a moderate preference to select partners in the same sexual activity group. The mixing between age groups was predominantly older men with younger women. They made the following vaccine assumptions: vaccination of 12-year-old girls with a series of three injections, 70% vaccine coverage, and 90% efficacy lasting for 10 years (with booster shot administration at the age of 22 years). They assumed 71% compliance with biennial screening.
Under these assumptions, they predicted that female-only vaccination would reduce lifetime risk of cervical cancer by 61.8%, whereas male-female vaccination would reduce it by 63.9% compared with screening alone. They reported that, as vaccine coverage increased, the number of cervical cancer cases decreased and that a male-female programme always resulted in less cervical cancer cases than a female-only programme, but the difference was only large when levels of female vaccine penetration were low. Taira et al.[37] also stated that focusing on vaccination of infants or 12-year-old girls led to approximately the same reduction in lifetime risk of cervical cancer and that delaying vaccination to the age of 18 years resulted in a 54.7% decrease in cervical cancer cases compared with no vaccination.
2.3 Dynamic Models
2.3.1 Hughes et al., 2002
Hughes et al.[26] developed a dynamic model looking at protection against HPV disease with the HPV vaccine in a population. The model had five states: (i) susceptible; (ii) infected; (iii) immune; (iv) effectively vaccinated with no experience of infection; and (v) effectively vaccinated but infectious because of a breakthrough infection. There was no CIN or cervical cancer state. They divided the population into three sexual activity groups and made the following assumptions about a monovalent HPV vaccine (against HPV-16): 90% vaccine coverage and 75% vaccine efficacy with a 10-year duration of protection; age at vaccination was not reported. With these parameters, a female-only programme reduced HPV prevalence by 30.2%, whereas male-female vaccination reduced it by 44.2%. They also estimated that targeting vaccination to high-risk women only decreased HPV prevalence by 11.6% and thus this strategy is less effective than vaccinating all women.
2.3.2 Barnabas et al., 2006
Barnabas et al.[38] used a dynamic model to explore the impact of vaccination against HPV-16 in Finland. They stratified their population into 17 groups according to age (<85 years) and into four other groups according to the rate of sexual partner change. Their dynamic model for the natural history of HPV-16 infection was divided into eight states: (i) susceptible; (ii) infected with HPV-16; (iii) immune against HPV-16; (iv) LSIL; (v) HSIL; (vi) ICC; (vii) cancer survivors; and (viii) death. They used data from the national Finnish screening programme to determine the percentage of women screened every 5 years between the ages of 30 and 60 years. Progression and regression rates of HPV used in the model were based on a review of studies on HPV infections. The authors assumed that HPV-16 accounted for 56% of ICC incidence. They also assumed vaccination of 15-year-old girls with a monovalent vaccine (before sexual debut) with 90% vaccine coverage, 100% efficacy and lifelong immunity. With these assumptions, they estimated that vaccination against HPV-16 and screening decreased the incidence of cervical cancer associated with HPV-16 by 92.8% compared with screening alone. They showed that male-female vaccination decreased the incidence of cancer by an additional 7%. Delaying vaccination to the age of 20 years resulted in a lower reduction in cervical cancer incidence compared with vaccination at 15 years (63% vs 92.8%, respectively). Decreasing the frequency of screening to every 10 years was also associated with a greater incidence of cancer (reduction of 85.7% vs 92.8%, respectively).
2.3.3 French et al., 2007
French et al.[39] used the dynamic model of Barnabas et al.[38] to further explore the impact of age at first sexual intercourse and age of vaccination on occurrence of HPV-16 and the associated cases of cervical cancer. The authors looked at different vaccination ages (12, 15, 18 and 21 years) and assumed 70% vaccine coverage, 100% efficacy and lifelong immunity. Screening was assumed to be initiated at age 25 or 30 years and continued up to 60 years at 5-year intervals. Once the full impact of vaccination would be reached, French et al.[39] estimated that the annual proportion of HPV-16-associated cervical cancer prevented would be 20% for vaccination at age 21 years, 40% at 18 years, 67% at 15 years and 68% at 12 years. The incremental reduction in the incidence of cancer when males were also vaccinated was 15.1%, 15.5% and 1% at age 12, 15 and 21 years, respectively. When a 3-year catch-up programme was added, the additional reduction in cervical cancer cases in the first 10 years after the start of vaccination was 15% when vaccination occurred at age 12 years, 18% at 15 years, 10% at 18 years and 6% at 21 years.
2.3.4 Elbasha et al., 2007
Elbasha et al.[40] recently developed a dynamic model to assess the epidemiological consequences and cost effectiveness of alternative quadrivalent HPV-6, -11, -16, -18 vaccination strategies. They divided their population into 17 age categories and divided these groups into three further subcategories based on sexual activity. In their model, the formation of sexual partnership was governed by a conditional probability sexual mixing matrix. Their dynamic model was divided into 15 states: (i) susceptible; (ii) infected with HPV-16, -18; (iii) infected with HPV-6, -11; (iv) immune against HPV-16, -18; (v) immune for HPV-6, -11; (vi) co-infected with HPV-6, -11, -16, -18; (vii) immune against HPV-6, -11, -16, -18; (viii) genital warts; (ix) CIN-1; (x) CIN-2; (xi) CIN-3; (xii) localized cancer; (xiii) regional cancer; (xiv) distant cancer; and (xv) death. In addition to looking at vaccination of 12-year-old females and males, they also investigated the impact of a catch-up programme for 12- to 24-year-old females and/or males. They assessed the impact of vaccination over a period of 100 years. They assumed 70% vaccine coverage, 90% effectiveness against HPV infections, 100% effectiveness against HPV-related diseases and lifelong protection. The reduction in HPV prevalence was not reported, whereas the incidence of cervical cancer with a quadrivalent vaccine against HPV-6, -11, -16, -18 and screening was reduced by approximately 75% compared with screening alone. The effect of vaccination on the reduction in incidence of HPV-16 and -18-related cervical cancer was more gradual than that for CIN-2 and -3 and for genital warts, for which the reduction occurred sooner. Vaccination and catch-up for males and females achieved a 97% reduction in cases of genital warts, and a reduction of 91% in both CIN-2, -3 and cervical cancer in females. With a 10-year duration of vaccine protection, the long-term reduction of genital wart incidence in men was 36%, whereas reduction of CIN-2, -3 and cervical cancer in women was 25% and 28%, respectively. The incidence of cervical cancer was not very sensitive to changes in vaccine efficacy; however, it was sensitive to vaccine coverage (lower impact with lower vaccine coverage).
2.3.5 Günther et al., 2008
Günther et al.[41] recently developed a model to determine transmission dynamics of HPV-16 and -18 using nine different states: (i) susceptible; (ii) exposed; (iii) infectious; (iv) immune; (v) LSIL; (vi) HSIL; (vii) cervical cancer; (viii) treatment; and (ix) death. Data from the British Columbia Centre for Disease Control and the British Columbia Cancer Agency Screening Program[42] were used to determine HPV prevalence, infection rates, development of SIL, cervical cancer, death rate and costs associated with screening and treatment. In the model, the population was divided into ten age groups and four other groups according to sexual activity. The sexual mixing pattern used by the authors modelled the preferences of older men for younger women on a background of within-group and random mixing. This model looked at the cost effectiveness of several different vaccination strategies: (i) school-based programme for 11-year-old girls (grade 6); (ii) school-based programme for 14-year-old girls (grade 9); (iii) combination programme for 11- and 14-year-old girls (grades 6 and 9); and (iv) school-based programme for 14-year-old girls and boys. The following assumptions were made by the authors: three doses of the vaccine would be administered by the school nurse, vaccine coverage of 85% in grade 6 and 80% in grade 9, vaccine efficacy of 100% against HPV-16, -18 with lifelong duration of protection. It was also assumed that 71% of women would undergo Pap smear testing every 2 years. Based on these assumptions, in the screening setting, the model estimated that HPV-16, -18 prevalence would be decreased by 84.4% with vaccination of 11-year-old girls as well as vaccination of 11-year-olds plus a 3-year catch-up programme for 14-year-old girls compared with screening alone. For a vaccination programme of 14-year-old females, HPV prevalence would be reduced to a lesser extent (74.5%). These reductions were associated with reductions in HPV-16, -18-related cervical cancer of 43.0%, 41.4% and 46.0% with 11-year-old, 14-year-old and combined 11- and catch-up 14-year-old programmes, respectively.
2.3.6 Insinga et al., 2007
The dynamic transmission model developed by Elbasha et al.[40] was used by Insinga et al.[43] to examine the potential health outcomes and cost effectiveness of the quadrivalent HPV vaccine in the Mexican population. The strategies used in the study were as follows: (i) no vaccination; (ii) vaccination of 12-year-old females; (iii) vaccination of 12-year-old males and females; (iv) vaccination of 12-year-old females, plus temporary 5-year catch-up programme for 12- to 24-year-old females; (v) vaccination of 12-year-old males and females plus temporary 5-year catch-up programme for 12- to 24-year-old females; and (vi) vaccination of 12-year-old males and females plus temporary 5-year catch-up programme for 12- to 24-year-old males and females. The model set the population size at 100 000 individuals and categorized heterosexual mixing of the population into 17 age groups. They assumed three doses would be administered to 12-year-old females and males, with a 70% coverage, which was in linear increments during the first 5 years following vaccine introduction. They assumed 70% vaccine coverage, 90% vaccine efficacy and lifelong protection. The most effective strategy was vaccination of 12-year-olds plus a temporary catch-up programme covering 12- to 24-year-old males and females. This strategy reduced HPV-related cervical cancer, high-grade cervical pre-cancer and genital warts by 84–98% 50 years after vaccine introduction. The effect of vaccination on the reduction of genital wart incidence following vaccination occurred sooner across all six strategies.
2.3.7 Regan et al., 2007
Regan et al.[44] developed a mathematical model to estimate the impact of vaccination on HPV-16 disease in Australia. The dynamic transmission model was stratified according to age, sex, level of sexual activity and HPV infection using a differential formulation. The population was assumed to be uniformly distributed between 0 and 85 years of age in a year-age band, whereas the population was time invariant. It was also assumed that the immune system responds independently to HPV type. A value of approximately 20% at the pre-vaccination equilibrium was obtained as the estimate of lifetime risk of HPV-16 infection. The model was used to estimate the expected reduction in HPV incidence and prevalence resulting from vaccination; the time span over which these reductions could be achieved; and the coverage required for HPV elimination. The model assumed 100% vaccine efficacy and lifelong vaccine protection. The model predicted that vaccinating 80% of 12-year-old girls would reduce HPV-16 prevalence by 60–100% in vaccinated females and by 7–31% in unvaccinated females. According to their model, if 80% of boys were vaccinated, there would be a reduction in HPV-16 prevalence of 74–100% in vaccinated females and 86–96% in unvaccinated females. In the absence of a catch-up programme, it would take 5–7 years to achieve 50% of the eventual reduction (if a 12-year-old female-only programme was assumed) but if the catch-up programme included 13- to 26-year-olds, the delay would be 2 years.
2.3.8 Kim et al., 2007
Kim et al.[45] created an open-cohort, age-structured (ages 0–90 years in yearly intervals) dynamic transmission model in which Brazilian males and females entered the susceptible pool at the age of 12 years. The model was calibrated using data from two large epidemiological studies[46–48] on HPV-16 and -18. This dynamic model evaluated the impact of HPV vaccination on HPV-16 and -18. Coverage was varied from 0% to 90% and the model evaluated both a girls-only programme and a boys-girls programme. The model showed the reduction in overall cancer risk was 14% when coverage was 25%, and 63% at 90% coverage.
2.3.9 Goldie et al., 2007
Goldie et al.[49] modified their previous US-based dynamic transmission model to evaluate cost effectiveness of the HPV-16 and -18 vaccines in Brazil, a country in which cervical cancer contributes to more life lost than AIDS or tuberculosis.[50] The authors used an empirically calibrated simulation model of cervical cancer to compare alternative approaches to cervical cancer prevention. The model was calibrated using likelihood base. A subset of good-fitting parameter sets was used to compare HPV-16 and -18 vaccination policies. Outcomes measured in the study included cervical cancer prevented. A monthly transition between mutually exclusive health states was used to represent the natural history of disease. The health states used were HPV infection status, CIN grade and stage of cancer. HPV types were categorized into four groups: (i) high-risk HPV-16; (ii) high-risk HPV-18; (iii) other high-risk types; and (iv) low-risk types. The model had girls entering at the age of 9 years, before sexual debut, and followed through their lifetime. Base-case parameters included 70% of the target population receiving three doses of the vaccine before age 12 years, 100% vaccine efficacy against HPV-16, -18 with lifelong immunity, cervical cytology screening and HPV DNA testing three times in a lifetime. Screening at ages 35, 40 and 45 years per lifetime with cytology had a mean reduction of 21.9% in cervical cancer, vaccination alone provided a 42.7% reduction and a combined approach of adolescent vaccination and screening of adult women yielded a mean reduction of 55.6% (range 47.7–64.8%) in cervical cancer, with a three visit per lifetime cytology and 60.8% (range 52.8–70.1%) reduction with two visits per lifetime for HPV DNA testing.
2.3.10 Goldhaber-Fiebert et al., 2007
Goldhaber-Fiebert et al.[51] developed a stochastic microsimulation of cervical cancer that distinguished different HPV types by their incidence, clearance, persistence and progression. The input parameters used in their model were sampled randomly from a uniform distribution and individual simulation on each parameter. They established multiple epidemiological targets, such as age-specific prevalence of HPV by type, through formal data synthesis and systematic review. They evaluated the performance of their model in terms of its external consistency and face validity by comparing model output and data from several large US screening studies not used in the parameterization or calibration of the natural history model. Their model evaluation results showed a model output that was consistent with the general shape of the decline in high-risk HPV prevalence for older age groups; however, for some parameterization, the model produced lower age-specific estimates than the observed data. In addition, their results showed that the expected reduction in lifetime risk of cancer with annual and biennial screening was approximately 76% and 69%, respectively. The reduction from vaccination alone was 75%. Combined vaccination and 5-yearly screening resulted in a mean reduction of 89% in cervical cancer incidence.
2.3.11 Chesson et al., 2008
Chesson et al.[52] used an incidence-based model to determine cost effectiveness of HPV vaccination in the US from a societal perspective. The analysis targeted HPV-18, -16, -11 and -6. The model assessed the health and economic effect of HPV-related outcomes in the absence of vaccination and determines how these effects changed over time with the introduction of an HPV vaccine. The strategy used was the existing Pap smear screening programme. The model assumed that the vaccine would be administered to 12-year-old females starting in year 1 through to year 100. Assumptions were 70% vaccine coverage, 100% vaccination efficacy, lifelong duration of protection and a vaccine cost of $US360 per dose. This analysis showed that, in a population model of a quadrivalent vaccine with no herd immunity benefit, the reduction in CIN, cervical cancer and genital warts was approximately 70%, 19% and 12% of averted cost, respectively.
3. Modelling Cost Effectiveness
Of the 22 mathematical models looking at health outcomes associated with HPV vaccination, 13 also evaluated vaccine cost effectiveness compared with the cytology-based Pap smear screening programme.[27,28,30,31,33–37,40,43,45,49,52] The assumptions used in these modelling studies and their outcomes in terms of epidemiological impact on HPV, cervical cancer incidence and cost effectiveness are shown in the supplementary material (see ‘ArticlePlus’ at http://pharmacoeconomics.adisonline.com).
All 13 studies included direct medical costs such as the cost of the vaccine, as well as costs for the management and treatment of cancer precursors and cervical cancer. Only Chesson et al.[52] included the costs associated with some non-cervical cancers (vaginal, vulvar, anal and head cancers). A number of investigators included costs associated with treatment of genital warts in their model and the impact of genital warts on quality of life (QOL).[33,35,36,40,44,52] For indirect costs associated with HPV vaccination, the studies by Goldie et al.,[30,49] Kim et al.[45] and Chesson et al.[52] accounted for patient (or parent) time associated with vaccination and screening and, therefore, they are the only authors who adopted a societal perspective for their base-case cost-effectiveness analysis. Kulasingam and Myers[28] evaluated the impact of parent time taken for three office visits for vaccination in their sensitivity analyses. All studies used an annual discount rate of 3% for both costs and QALYs. The HPV types targeted by the vaccine were different across the studies (see previous section). In addition to the assumptions described in the previous section, each of these studies included various assumptions for the cost of vaccination and used different values based on various sources of information to determine costs for the management of HPV-related infections and cervical cancer.
3.1 Markov Models
3.1.1 Sanders and Taira, 2003
Sanders and Taira[27] used direct costs for screening and treatment of SIL and cervical cancer, which were based on Medicare average reimbursement rates. Utilities for these health states were based on data from the Institute of Medicine Vaccines for the 21st Century, which used committee-consensus Health Utility Indices levels for relevant health states.[53] They assumed a cost of $US300 (year 2001 values) per three-dose vaccine series and $US100 per booster shot (required every 10 years). Based on these costs, they projected a cost of $US32 066 per LY gained and $US22 755 per QALY gained with Pap smear screening and vaccination against 13 high-risk HPV types versus screening alone. In sensitivity analyses, the authors estimated that vaccine efficacy was the parameter with the greatest impact on their results. They also estimated that, with lifelong immunity, the incremental cost per QALY would improve to $US12 682. Vaccination of 15- versus 12-year-old girls would cost $US40 440 per QALY gained compared with screening alone. With 100% compliance with Pap testing (instead of 71%), the incremental cost per QALY would be $US33 218. Finally, the authors estimated a vaccination cost of $US37 752 per QALY gained when the annual discount rate was 5% (instead of 3%).
3.1.2 Kulasingam and Myers, 2003
Kulasingam and Myers[28] used direct medical costs for screening and diagnoses of CIN and cervical cancer, which were estimated from MEDSTAT, the National Ambulatory Medical Care Survey and Medicare Data. Vaccine cost was assumed to be $US200 for three doses (year 2001 values). The authors did not use health-related QOL (HR-QOL) data in their base-case analysis, but they did report that they used utilities derived from the literature in their sensitivity analyses. Based on these, they estimated a cost of $US92 677 per LY gained with biennial screening starting at 18 years and vaccination against 70% of high-risk HPV types compared with screening alone. The authors focused their sensitivity analyses on the strategy that appeared to be the most attractive: vaccination plus screening every 2 years beginning at the age of 24 years. Based on these analyses, they projected that the results were most influenced by age at vaccination (≤15 years old), HPV types covered by the vaccine (>70% of high-risk types), vaccination efficacy (>92%) and duration of immunity (>14 years).
3.1.3 Goldie et al., 2004
Goldie et al.[30] used a societal perspective for their base-case cost-effectiveness analysis. For vaccination, they included the costs of three brief clinic visits, surveillance and educational costs as well as patient (or parent) time. The cost per vaccination series was $US377 (year 2002 values). Direct medical costs for screening and treatment of CIN and cervical cancer were derived from previously published data. Utilities associated with non-cancer states were based on the Cost-Effectiveness in Health and Medicine, [54] whereas utilities associated with time spent in cancer states were derived from committee-consensus utility estimates by the Committee to Study Priorities for Vaccine Development (Institute of Medicine).[53] With these assumptions, they achieved a cost of $US24 300 per QALY for vaccination (plus screening) against HPV-16 and -18 compared with screening alone. In sensitivity analyses, their results were most sensitive to (i) alternative assumptions about the duration of vaccine efficacy; (ii) proportion of persistent HPV in women aged >30 years attributable to newly acquired HPV infection versus reactivation of infection acquired in earlier adulthood; and (iii) pattern of screening (age at initiation, frequency costs for follow-up of abnormal results). They also estimated that decreasing vaccination efficacy to 70% (vs 90%) was associated with an ICER of $US33 700 per QALY compared with screening alone.
The authors reported that, provided the vaccine is at least 70% effective, vaccination at the age of 12 years, combined with cytologic screening every 3 years beginning at the age of 25 years, is more effective than the current screening programme, provides a 92% reduction in cervical cancer incidence and costs approximately $US50 000 per QALY. The cost effectiveness of vaccination also increased with decreasing screening compliance. The authors also varied sensitivity of cytological screening type and estimated that, when the sensitivity of conventional cytological screening was reduced to <50%, liquid-based cytological screening was more cost effective.
3.1.4 Brisson et al., 2007
Brisson et al.[33] used a third-party payer perspective for their cost-effectiveness analysis. They are the only investigators who compared the quadrivalent and bivalent vaccine with a no-vaccination programme. The cost per vaccination series (both bivalent and quadrivalent vaccines) was $Can400. Direct medical costs for screening and treatment of CIN and cervical cancer were derived from previously published US data and converted to $Can. Under base-case assumptions, vaccinating 100 000 girls resulted in 1400 discounted QALYs saved over their lifetime using a bivalent vaccine, and 1800 using the quadrivalent vaccine. This was estimated to result in approximately $Can31 000 (80% CI 15 000, 55 000) per QALY gained for the bivalent vaccine and $Can21 000 (80% CI 11 000, 33 000) per QALY gained for the quadrivalent vaccine (year 2005 values). In sensitivity analyses, their results were most sensitive to age at vaccination, duration of vaccine protection, vaccine cost and QALY lost due to genital warts, and were least sensitive to the medical costs. In terms of vaccine cost, the authors suggest that the cost per course of the bivalent and quadrivalent vaccines would probably not be identical. Assuming the quadrivalent costs $Can400 per course, Brisson et al.[33] estimated that the cost per course of the bivalent vaccine must be approximately $Can295 (80% CI 235, 347) in order for the vaccines to produce equivalent cost-utility ratios (i.e. < $Can40 000 per QALY).
3.1.5 Kulasingam et al., 2007
Kulasingam et al.[34] obtained their cost of screening, follow-up and treatment from the Medicare Australia Schedule Fees. All costs were measured in Australian dollars ($A), year 2005 values. The cost of the school delivery programme was based on the Municipal Association of Victoria’s Cost of Victorian Local Government Immunisation Service and the GP cost was based on the schedule fee. Utilities estimates reported in Myers et al.[58] were used for the different health states, and a discount rate of 5% was assigned for the base case. The incremental cost per LY gained for adding vaccination was $A51 103 ($A18 735 per QALY), assuming a vaccine cost of $A115. The ICER was relatively insensitive to assumptions about the specificity of the Pap smear test, but was sensitive to assumptions about the duration of vaccine efficacy and the need for a booster dose in order to produce lifelong immunity ($A68 158 per LY gained; $A24 988 per QALY). If the cost of vaccine was reduced to $A100, the ICER decreased to $A42 567 per LY gained or $A15 606 per QALY. Incorporating herd immunity resulted in a more attractive ICER ($A36 343 per LY gained; $A13 316 per QALY).
3.1.6 Kulasingam et al., 2008
Kulasingam et al.[35] obtained costs for screening, diagnosis and treatment for cervical cancer and genital warts from previously published studies. Costs used were inflated to £ (year 2005 values) using the Hospital and Community Services pay and price index.[55] Economic analysis indicated that screening only had an ICER of £11 156 per QALY. Combined screening and vaccination had an ICER of £21 059 per QALY and £34 687 per LY saved. The sensitivity analysis showed that the results were sensitive to duration of immunity. For instance, if a booster was needed to achieve lifetime protection (given at 22 years and to 50% of women), the ICER was £26 782 per QALY and £44 114 per LY saved. The results were also sensitive to the discount rate — when a 1.5% rate was used, the ICER decreased to £9653 per QALY. Varying costs of screening, diagnosis, treatment and cost of vaccine over a wide range had a moderate impact on the cost effectiveness of vaccination.
3.1.7 Bergeron et al., 2008
Bergeron et al.[36] assessed the cost effectiveness of an HPV vaccine from a third-party payer and direct healthcare cost perspective. Two vaccine costs were used: €264 for the direct healthcare cost perspective and €406.8 for the third-party payer perspective. Various costs associated with screening and vaccination were detailed. Utility values used were derived using the time trade-off technique. The utilities used were derived from a study conducted among college-aged students in the US. The change in LY gained for third-party payer and direct healthcare costs were 0.0154 and 0.0152, respectively, whereas change in QALYs were 0.0228 and 0.0225 for third-party payer perspective and direct healthcare cost perspectives, respectively. The ICER for the screening and vaccination programme versus screening alone was €12 429 per LY gained and €8408 per QALY for third-party payers. An ICER of €20 455 per LY gained and €13 809 per QALY gained were obtained from the direct healthcare cost perspective. Thus, the authors concluded that the quadrivalent vaccine plus current screening was cost effective as it was below the threshold of €50 000 per QALY gained.
3.2 Hybrid Models
3.2.1 Taira et al., 2004
Taira et al.[37] estimated costs and utilities from previous analyses.[27] Since they used a hybrid model with a dynamic component, they could assess the impact of herd immunity on the cost effectiveness of HPV vaccination (in contrast to Sanders and Taira,[27] who only used transition-state modelling). They achieved a cost per QALY gained of $US14 583 with a female-only vaccination programme against HPV-16 and -18 plus screening compared with screening alone (which is lower than $US22 755 per QALY gained estimated without accounting for herd immunity). They estimated the cost-effectiveness ratio of male-female vaccination to be $US442039 per QALY gained compared with female-only vaccination. The authors reported that vaccination of boys in addition to girls was cost effective at intermediate levels of vaccine efficacy. They also stated that, considering that vaccine efficacy waned over 10 years and no booster was administered, vaccination at age 18 years would be more cost effective than at age 12 years. In this scenario, vaccination of 18-year-old males and females cost $US57 795 per QALY gained compared with female-only vaccination. However, when two booster shots were administered at 5-year intervals, vaccination at age 12 years was more cost effective than at 18 years of age. However, in this scenario, vaccination of boys was not cost effective (cost per QALY gained of $US388 368; year 2004 values).
3.3 Dynamic Models
3.3.1 Elbasha et al., 2007
Elbasha et al.[40] evaluated the cost effectiveness of a quadrivalent vaccine. The authors derived direct medical costs for screening and treatment of CIN, genital warts and cervical cancer from administrative claims and previously published data, whereas health utilities were derived from previously published data only. Vaccine cost was assumed to be $US360 for three doses (year 2005 values). Female-only vaccination with a quadrivalent vaccine against HPV-6, -11, -16, -18 resulted in an incremental cost of $US2964 per QALY compared with screening alone. Adding vaccination of 12-year-old boys was not cost effective, whereas adding a catch-up programme for 12- to 24-year-old females cost $US4666 per QALY gained. Vaccination of 12-year-old boys and girls with a female-only or a male-female catch-up programme had an incremental cost per QALY of $US41 803 and $US45 056, respectively. In their sensitivity analysis, the authors obtained the following ICERs for a female-only vaccination programme: not cost effective with a 10-year vaccine efficacy duration; $US2094 per QALY with 100% vaccine efficacy; $US4273 with 74% vaccine efficacy; $US2636 with 50% vaccine coverage; and $US3449 with 90% vaccine coverage.
3.3.2 Insinga et al., 2007
Insinga et al.[43] used direct medical costs based on a recent micro-costing study conducted within Mexico’s National Public Health Institute. Costs were estimated from the perspective of the Mexican healthcare system. They assumed that the cost per case of genital warts in Mexico was relative to that for CIN-3: Mexican pesos ($Mex)1441 (year 2005 values). They also assumed a cost of $US240 for the three-dose vaccine series. Non-medication costs, such as patient lost wages and transportation, were not included in the study. All costs were discounted at a 3% annual rate. With the different strategies assessed, a vaccination programme for 12-year-old females was found to have an ICER of $US2719 per QALY. The most effective strategy involving male-female vaccination at age 12 years, and a temporary catch-up programme for both sexes aged 12–24 years gave an ICER of $US16 702 per QALY when compared with vaccination of both sexes at age 12 years with a catch-up programme for age 12- to 24-year-old females.
3.3.3 Kim et al., 2007
Kim et al.[45] used a societal perspective and discounting of 3% in their model. They varied coverage from 0% to 90% in both sexes, and a cost per vaccinated individual of $US25–400 for the three doses. At a cost of $US25 per vaccinated individual, vaccinating pre-adolescent girls was cost saving compared with no vaccination, at all coverage levels. When this cost increased to $US50, vaccination was no longer cost saving but was less than $US200 per LY gained. Including boys increased the cost per LY gained, which ranged from $US810 to $US18 650 per LY gained (year 2000 values) depending on the vaccination coverage rates.
3.3.4 Goldie et al., 2007
Goldie et al.[49] performed their analysis from a societal perspective. Thus, screening, diagnosis and treatment costs were categorized as direct costs but patient time spent travelling and receiving care were also included as indirect costs. Cost data were presented in international dollars (I$), year 2000 values. If costs were not available from Brazil, the authors used data from other countries. They used a composite cost range of I$25–450 as their cost per vaccinated woman (this included the vaccine cost but also cost of wastage and supplies as well as administration costs). Their analysis showed that, if the cost per vaccinated woman was equal to or below I$50, then screening alone three times per lifetime was dominated by vaccination alone. However, vaccination alone was cost saving when cost per vaccination was I$25 or less compared with no intervention, and cost effectiveness of a combined vaccination/screening programme ranged from I$200 to I$700 per LY gained, depending on the choice of screening test. Numerous sensitivity analyses were performed and the single most influential factor was the cost of the vaccine. The authors concluded that, provided the cost of vaccination was I$25 or less (which would mean a vaccine cost of I$5), then HPV vaccination before 12 years of age, followed by screening three times per lifetime between the ages of 35 and 45 years would be cost effective in Brazil.
3.3.5 Chesson et al., 2008
Chesson et al.[52] adopted a societal perspective and their costs were discounted at 3% (year 2005 values). The cost of vaccination was $US360 per series (year 2005 values). Under base-case assumptions, the cost per QALY gained for vaccinating 12-year-old girls, assuming a population model that accounted for herd immunity, and using a quadrivalent vaccine with an endpoint of cervical cancer was $US5336 per QALY (using a bivalent vaccine it was $US10 318 per QALY). Inclusion of costs associated with anal, vaginal, vulvar and oropharyngeal cancers decreased the cost effectiveness to $US3906 per QALY and $US7848 per QALY for the quadrivalent and bivalent vaccines, respectively. The sensitivity analysis using the best- and worst-case scenarios showed a cost per QALY ranging from <$US0 to $US122 976; however, much of this was due to changes in discount rate and the time horizon.
4. Discussion
Randomized controlled trials for the HPV vaccines have shown a high level of efficacy in reducing the risk of HPV acquisition, CIN development associated with HPV-16, -18 and development of HPV-6, -11-associated genital warts. Various efficacy studies[13–19] have shown a >95% efficacy rate (95% CI 86.4, 100). The vaccine is recommended for females aged 9–26 years, and various countries are considering recommending the vaccine in elementary schools (e.g. Canada) or have already done so (US, Australia).
Although clinical trials provide important information around vaccine efficacy, decision makers need additional data in order to make a policy decision for their jurisdictions. For this, mathematical modelling and economic analyses are helpful to project the long-term benefits of HPV vaccination programmes in terms of HPV disease, precancerous lesions and cervical cancer incidence as well as programme cost effectiveness.
Two prior review articles have been published on the cost effectiveness of HPV vaccination.[56,57] Both reviews included only four to six studies as they were conducted around 2005. In addition, the review by Ortega-Sanchez et al.[56] examined economic studies of all adolescent vaccines (i.e. hepatitis, HPV, influenza, meningococcal and pertussis) and not just HPV vaccine. Hence, not much detail was included in their paper.
Our systematic analysis showed that, although the studies used slightly different baseline assumptions and modelling designs, their findings were remarkably similar. As shown in the supplementary material, static Markov modelling studies found that the use of HPV vaccine in 12-year-old girls would reduce the incidence of HPV infections by approximately 13%, CIN-1 by 21–24% and CIN-2, -3 by 43–58%. The first modelling studies conducted in 2003[28,27] showed a reduction in cervical cancer cases of 15% and 20%, respectively. However, the studies conducted thereafter[29–31,33–36] showed a greater reduction of up to 58%. Cervical cancer-related death was evaluated by Sanders and Taira[27] and Kulasingam et al.,[35] who showed a 21% and 58% reduction, respectively, with the introduction of an HPV vaccine. In addition, studies by Brisson et al.[33] and Kulasingham et al.[35] reported a 86% and 67% reduction, respectively, in cases of genital warts with the introduction of the quadrivalent vaccine. The hybrid and dynamic models predicted an approximately 95% reduction in HPV infections, and a 62–93% reduction in cervical cancer cases with female-only vaccinations but 64–91% reductions in cervical cancer cases if vaccinating girls and boys[26,37–41,43–45,49,51,52] and 14–63% if the vaccine coverage ranged from 25–90%.[45] Four of the investigators[33,35,40,43] evaluated the impact of the vaccine on HPV-6, -11 (which cause genital warts) in their models and showed an approximately 83% reduction in cases of genital warts if vaccinating girls only[40] and 93–97% reduction if vaccinating both girls and boys.[33,40,43,45]
Studies evaluating the cost effectiveness of an HPV vaccine found that a vaccination programme for girls would be cost effective. In general, the studies using static Markov models, as shown in the supplementary material, found the cost per LY gained ranged from approximately $US32 000 to $US93 000 when the HPV vaccine was introduced for 12-year-old girls compared with the current screening programmes, while the incremental cost per QALYs in these studies ranged from $US23 000 to $US31 000.[27,28,30,33,34] Two studies[35,36] assessed cost per LYs in other currencies. Kulasingam et al.[35] found the cost per LY gained was £34 687 and the cost per QALY was £21 059. Bergeron et al.[36] showed that the cost per LY gained was €20 455 and that cost per QALY was €13 809. The dynamic models showed a lower ICER for the vaccine, ranging from approximately $US3000 to $US15 000 per QALY for a girls-only programme.[37,40,43,52,56] While one study[37] found that the cost of introducing a vaccination programme for girls and boys would be at an incremental cost of approximately $US440 000 compared with a girls-only programme; other models found a boys and girls programme to cost approximately $US17 000–42 000 per QALY.[37,40]
There are a number of limitations associated with these modelling studies. Studies using static Markov modelling did not account for herd immunity, and therefore they are more likely than the dynamic models to have underestimated the cost effectiveness of HPV vaccination. The dynamic transmission models did not consider homosexual and bisexual transmission of HPV infection and as such, are likely to have underestimated the cost effectiveness of the vaccine in men. An assumption of high screening compliance, such as that adopted by Kulasingam and Myers[28] and Goldie et al.,[29] is also likely to have underestimated the cost effectiveness of vaccination as compared with screening only. The large variation in QOL weights across the studies also further complicates the incremental cost per QALY comparisons. Since these studies made assumptions regarding HPV vaccine characteristics, it is important to reassess the cost-effectiveness results of these studies once data regarding vaccine efficacy and duration of immunity becomes available from long-term clinical trials. This is particularly important with regard to duration of immunity secondary to vaccination, since all the studies found that cost-effectiveness results were sensitive to this parameter. Some of the models included a booster dose as a model parameter. However, there is no trial-based evidence showing that boosters are effective for reducing the risk of HPV infection or CIN, nor has there been regulatory approval or recommendation on the use of the vaccine for booster doses. Thus, studies using this assumption may be overestimating cost effectiveness of the HPV vaccine.
By failing to capture the full impact of the HPV vaccine on the avoidance of other diseases beyond cervical cancer, most of the studies are likely conservative in their estimates of the cost effectiveness of vaccination. Most of these studies did not include the impact of costs and utilities associated with genital warts and its management. According to the results of Elbasha et al.,[40] who examined such an impact, the cost effectiveness of HPV vaccination has likely been underestimated in those studies not considering the effect of genital warts on costs and utilities. Similarly, many of the studies did not include non-cervical cancer costs and utilities, and their inclusion, as with genital warts, would favour the cost effectiveness of the vaccine.
Kulasingam et al.[35] is one of the studies that clearly stated type-specific progression rates of relevant HPV serotypes. A few studies made reference to the type-specific progression rates in their supplementary paper. Taira et al.[37] did model type-specific HPV-16 and -18 transition probabilities, but they did not state specific values in their manuscript. Goldie et al.[49] also modelled high- and low-risk HPV types but did not report differences in transition probabilities by HPV type. Another limitation of these studies is that, except for Goldie et al.,[30] most of the authors did not examine the effect of reactivation of latent infections or cross protection in older women. Finally, a few studies (e.g. Goldhaber-Fiebert et al.,[51] who gave explicit detail on their model parameterization and validation process), reported a model calibration process with type- and age-specific data on rates of HPV infection, CIN and cervical cancer. In the model-validation process they all found their predicted epidemiological outcomes, such as age-specific annual incidence of cervical cancer, to be similar to observed data. However, the lack of reporting about the validation process in most HPV modelling studies makes it hard to determine if the models provide an accurate description of epidemiological patterns expected prior to a vaccination programme and if they can be used to predict future incidence and outcomes associated with introducing the vaccine.
5. Conclusions
We have begun a new era in cervical cancer disease, in which protection is possible through vaccination programmes. The models show that vaccination with HPV vaccines will have a substantial impact on the epidemiology of this disease. HPV incidence, precancerous lesions and cervical cancer rates will be significantly reduced over time. The extent of this reduction will be dependent upon vaccination coverage rates, whether a girls-only, girls-and-boys or girls plus catch-up programme is used. All models showed that the HPV vaccine in females is cost effective, as they produced a cost per QALY ratio below the traditionally used cut-off of $US50 000 per QALY. In general, the dynamic models that included the concept of herd immunity showed lower cost-effectiveness ratios than the Markov models.
References
Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55: 244–65
Goodman A. Primary vaginal cancer. Surg Oncol Clin N Am 1998; 7: 347–61
Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol 2005; 106: 1319–26
Dianzani C, Calvieri S, Pierangeli A, et al. Identification of human papilloma viruses in male dysplastic genital lesions. New Microbiol 2004; 27: 65–9
McKaig RG, Baric RS, Olshan AF. Human papillomavirus and head and neck cancer: epidemiology and molecular biology. Head Neck 1998; 20: 250–65
Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol 1995; 33: 2058–63
Derkay CS, Darrow DH. Recurrent respiratory papillomatosis of the larynx: current diagnosis and treatment. Otolaryngol Clin North Am 2000; 33: 1127–42
Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118: 3030–44
Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide. IARC CancerBase No.5, Version 2.0. Lyon: IARC Press, 2004
Muñoz N, Bosch FX, De Sanjosé S, et al., International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518–27
Merck Frosst. Gardasil®: quadrivalent human papillomavirus (types 6,11,16,18) recombinant vaccine [product monograph]. Date of revision: 2007 Jun 26 [online]. Available from URL: http://www.merckfrosst.ca/mfcl/en/corporate/products/gardasil.html [Accessed 2008 Jun 18]
GlaxoSmithKline. Cervarix™: human papillomavirus (types 16,18) recombinant adjuvanted, adsorbed vaccine [product monograph]. Date of revision: 2007 Sep [online]. Available from URL: http://www.gsk.com/media/pressreleases/2006/2006_06_05_GSK847.htm
Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 2002; 347: 1645–51
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol 2006; 107: 18–27
Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271–8
Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 2006; 24: 5571–83
Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95: 1459–66
Harper DM, Franco EL, Wheeler CM, et al., HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–55
Harper DM, Franco EL, Wheeler C, et al., GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364: 1757–65
Barr E, Tamms G. Quadrivalent human papillomavirus vaccine. Clin Infect Dis 2007; 45 (5): 609–7
Garnett GP, Kim JJ, French K, et al. Chapter 21: modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24 Suppl. 3: S178–86
Dasbach EJ, Elbasha EH, Insinga RP. Mathematical models for predicting the epidemiologic and economic impact of vaccination against human papillomavirus infection and disease. Epidemiol Rev 2006; 28: 88–100
Newall AT, Beutels P, Wood JG, et al. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7: 289–96
Goldie S, Goldhaber-Fiebert JD, Garnett GP. Chapter 18: public health policy for cervical cancer prevention. The role of decision science, economic evaluation, and mathematical modeling. Vaccine 2006; 24 Suppl 3: S155–63
Drummond MF, Richardson WS, O’Brien BJ, et al. User’s guides to the medical literature: XII. How to use an article on economic analysis of clinical practice: A. Are the results of study valid? JAMA 1997; 207 (19): 1552–7
Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology 2002; 13: 631–9
Sanders GD, Taira AV. Cost effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9: 37–48
Kulasingam SL, Myers ER. Potential health and economic impact of adding human papillomavirus vaccine to screening programs. JAMA 2003; 290: 781–9
Goldie SJ, Grima D, Kohli M, et al. A comprehensive natural history model of HPV infection and cervical cancer to estimate the clinical impact of a prophylactic HPV-16/18 vaccine. Int J Cancer 2003; 106: 896–904
Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004; 96: 604–15
Kohli M, Ferko N, Martin A, et al. Estimating the long-term impact of a prophylactic human papillomavirus 16/18 vaccine on the burden of cervical cancer in the UK. Br J Cancer 2007; 96: 143–50
UK Department of Health. Cervical screening program, England: 2003–04. Statistical Bulletin 2004/20 [online]. Available from URL: http://www.dh.gov.UK/assetRoot/04/09/63/75/04096375.pdf [Accessed 2008 Dec 30]
Brisson M, Van de Velde N, De Wals P, et al. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007; 25: 5399–408
Kulasingam S, Connelly L, Conway E, et al. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sexual Health 2007; 4: 165–75
Kulasingam SL, Benard S, Barnabas RV, et al. Adding a quadrivalent human papillomavirus vaccine to the UK cervical cancer screening programme: a cost-effectiveness analysis. Cost Eff Resour Alloc 2008; 6 (1): 4–15
Bergeron C, Largeron N, McAllister R, et al. Cost-effectiveness analysis of the introduction of a quadrivalent human papillomavirus vaccine in France. Int J Technol Assess Health Care 2008; 24 (1): 10–9
Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004; 10: 1915–23
Barnabas RV, Laukkanen P, Koskela P, et al. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Med 2006; 3: e138
French KM, Barnabas RV, Lehtinen M, et al. Strategies for the introduction of human papillomavirus vaccination: modelling the optimum age- and sex-specific pattern of vaccination in Finland. Br J Cancer 2007; 96: 514–8
Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13: 28–41
Günther OP, Ogilvie G, Naus M, et al. Protecting the next generation: what is the role of the duration of human papillomavirus vaccine-related immunity? J Infect Dis 2008; 197 (12): 1653–61
BC Cancer Agency. 2005 annual report. Vancouver (BC): The Cervical Cancer Screening Program, 2005 [online]. Available from URL: http://www.bccancer.bc.ca/NR/rdonlyres/34F5A494-73CD-4A7C-908A-D34F9E7B1F09/15237/2005ccsp_annual_reportFINAL.pdf [Accessed 2007 Oct 24]
Insinga RP, Dasbach EJ, Elbasha EH, et al. Cost-effectiveness of quadrivalent human papillomavirus (HPV) in Mexico: a transmission dynamic model-based evaluation. Vaccine 2007; 26: 128–39
Regan DG, Philp DJ, Hocking JS, et al. Modelling the population-level impact of vaccination on the transmission of human papillomavirus type 16 in Australia. Sexual Health 2007; 4: 147–63
Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. Br J Cancer 2007; 97: 1322–8
Measure DHS. Demographic and health surveys [online]. Available from URL: http://www.measuredhs.com/ [Accessed 2007 Jan 19]
United Nations Population Division. World population prospects: the 2004 revision population database [online]. Available from URL: http://www.un.org/esa/population/publications/WPP2004/World_Population_2004_chart.pdf [Accessed 2007 Jan 19]
US Census Bureau. Population estimates program. Washington, DC: US Census Bureau, Population Division, 2000 [online]. Available from URL: http://www.census.gov/popest/datasets.html [Accessed 2007 Sep 24]
Goldie SJ, Kim JJ, Kobus K, et al. Cost-effectiveness of HPV 16, 18 vaccination in Brazil. Vaccine 2007 Aug 14; 25 (33): 6257–70
Yang BH, Bray FI, Parkin DM, et al. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. Int J Cancer 2004; 109 (3): 418–24.
Goldhaber-Fiebert JD, Stout NK, Ortendahl J, et al. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr 2007; 5: 11
Chesson HW, Ekwueme DU, Saraiya M, et al. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg Infect Dis 2008 Feb; 14 (2): 244–51
Stratton KR, Durch J, Lawrence RS. Vaccines for the 21st century: a tool for decision making. Institute of Medicine (US): Committee to Study Priorities for Vaccine Development. Washington, DC: National Academy Press, 2000
Gold MR, Siegel JE, Russel LB, et al., editors. Cost-effectiveness in health and medicine. New York Oxford University Press, 1996
Curtis L, Netten A. Unit costs of health and social care 2005. Kent (UK): University of Kent, PSSRU, 2005
Ortega-Sanchez IR, Lee GM, Jacobs RJ, et al., Working Group on Leading Economic Issues for New Vaccine for Adolescents. Projected cost-effectiveness of new vaccines for adolescents in the United States. Pediatrics 2008; 121 Suppl. 1: S63–78
Newall AT, Beutels P, Wood JG, et al. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007 Apr; 7 (4): 289–96
Myers ER, Green S, Lipkus I. Patient preferences for health states related to HPV infection: visual analog scales versus time trade off elicitation. Proceedings of the 21st International Papillomavirus Conference; 2004 Feb 20–27; Mexico City
Acknowledgements
G. Ogilvie has previously received grants from Merck and Co. for an investigator-driven study on prevalence of HPV in British Columbia, Canada. The other authors have no conflicts of interest that are directly relevant to the content of this review. No sources of funding were used to assist in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Marra, F., Cloutier, K., Oteng, B. et al. Effectiveness and Cost Effectiveness of Human Papillomavirus Vaccine. Pharmacoeconomics 27, 127–147 (2009). https://doi.org/10.2165/00019053-200927020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200927020-00004