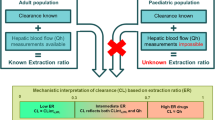
Abstract
The pharmacokinetics and pharmacodynamics of drugs are different in adult and paediatric populations, the latter being particularly heterogeneous. These differences in pharmacokinetics and pharmacodynamics justify specific studies but raise a number of ethical and practical issues. The main practical difficulties to circumvent while performing clinical studies in children are the invasiveness of the procedures and the obstacles to patient recruitment. The invasiveness related to pain/anxiety and blood loss precludes the performance of classical pharmacokinetic studies in children in many instances, particularly in neonates and infants. Population approaches, which rely on pharmacokinetic-pharmacodynamic modelling, are particularly appealing in paediatric populations because these models can cope with sparse data. The relevance of population approaches to investigation of the dose-concentration-effect relationships and to qualitative/quantitative assessment of factors that may explain interindividual variability has already been emphasized.
The aims of this review are to summarize the currently available literature on population pharmacokineticpharmacodynamic studies in children and to discuss a number of recent methodological developments that may facilitate the evaluation of drugs in this population by alleviating invasiveness and, subsequently, facilitating recruitment of patients. The present survey confirms that population approaches in paediatrics have already reached a large audience and that they are mostly used for analysis of sparse data. However, pharmacokineticpharmacodynamic studies in children are still scarce. New classes of models may extend the scope of the use of population models in paediatrics. Kinetic-pharmacodynamic models, where use of the term ‘kinetic’ rather than ‘pharmacokinetic’ emphasizes the absence of pharmacokinetic data, are indirect models where the (unobserved) drug kinetics are described by a single compartment involving a single rate constant. These models, which alleviate the need for blood samples used for the measurement of drug concentration, may be very useful in paediatric studies. Physiological and physiopathological models also have potential applications but require further development. Because the number of measurements in a single individual needs to be limited in children, it is crucial to optimize the design of the experiment in order to avoid inaccurate and unreliable results. In this review, formal optimization and simulation to evaluate a design are presented, and specific problems raised by the application of these techniques in paediatrics are addressed. Finally, the related technique of clinical trial simulation and its applications are presented and discussed.




Similar content being viewed by others
References
Steinbrook R. Testing medications in children. N Engl J Med 2002; 347: 1462–70
Rosato J. The ethics of clinical trials: a child’s view. J Law Med Ethics 2000; 28: 362–78
American Academy of Pediatrics Committee on Drugs. Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics 1995; 95: 286–94
Jong GW, Vuto AG, de Hoog M, et al. Unapproved and off-label use of drugs in a children’s hospital [letter]. N Engl J Med 2000; 343: 1125
Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000; 320: 79–82
Treluyer JM, Berger JF, Leclerc F, et al. Use of off-label and unlicensed drugs in neonatal and paediatric intensive care in France [abstract no. 46A]. Pediatric Academic Societies Annual Meeting; 1999 May 1–4; San Francisco (CA)
Bücheier R, Schwab M, Mörike K, et al. Offlabel prescribing to children in primary care in Germany: retrospective cohort study. BMJ 2002; 324: 1311–2
Schirm E, Tobi H, de Jong-van den Berg LT. Unlicensed and off label drug use by children in the community: cross sectional study. BMJ 2002; 324: 1312–3
Jong GW, Eland IA, Sturkenboom MCJM, et al. Unlicensed and off-label prescription of drugs to children: population based cohort study. BMJ 2002; 324: 1313–4
Chalumeau M, Treluyer JM, Salenave B, et al. Off label and unlicensed drug use among office-based paediatricians. Arch Dis Child 2000; 82: 502–5
O’Donnell CPF, Stone RJ, Morley CJ. Unlicensed and off-label drug use in an Australian neonatal intensive care unit. Pediatrics 2002; 110: e52
Nahata MC. Lack of pediatrie drug formulations. Pediatrics 1999; 104 Suppl.: 607–9
Kearns GL, Abdel-Rahman S, Alander SW, et al. Developmental pharmacologydrug disposition, action, and therapy in infants and children. N Eng J Med 2003; 349: 1157–67
US FDA. Specific requirements on content and format of labeling for human prescription drugs; revision of “pediatric use” subsection in the labeling; final rule [FR doc 94-30238; online]. Rockville (MD): US FDA, 1994 Nov 15. Available from URL: http://frwebgatel.access.gpo.gov/cgi-bin/waisgate.cgi?WAISdocID=386043433490+0+0+0WAISaction=retrieve [Accessed 2008 Feb 19]
Gao Y, Pons G, Rey E, et al. Could saliva stand for plasma in theophylline monitoring in asthmatic children? Still a controversial problem. Fundam Clin Pharmacol 1992; 6(4–5): 191–6
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: general principles. Eur J Pediatr 2006; 165(11): 741–6
Meibohm B, Läer S, Panetta JC, et al. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J 2005; 7(2): E475–87
Vozeh S, Steimer JL, Rowland M, et al. The use of population pharmacokinetics in drug development. Clin Pharmacokinet 1996; 30(2): 81–93
Samara E, Granneman R. Role of population pharmacokinetics in drug development: a pharmaceutical industry perspective. Clin Pharmacokinet 1997; 32(4): 294–312
Jackson KA, Rosenbaum SE. The application of population pharmacokinetics to the drug development process. Drug Dev Ind Pharm 1998; 24(12): 1155–62
Sun H, Fadiran EO, Jones CD, et al. Population pharmacokinetics: a regulatory perspective. Clin Pharmacokinet 1999; 37(1): 41–58
Sheiner L, Wakefield J. Population modelling in drug development. Stat Methods Med Res 1999; 8(3): 183–93
US FDA Center for Drug Evaluation and Research. Guidance for industry: population pharmacokinetics [online]. Rockville (MD): US FDA, 1999 Feb. Available from URL: http://www.fda.gov/CDER/guidance/1852fnl.pdf [Accessed 2008 Feb 19]
Sheiner LB, Beal SL, Sambol NC. Study designs for dose-ranging. Clin Pharmacol Ther 1989; 46(1): 63–77
Brendel K, Dartois C, Comets E, et al. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet 2007; 46(3): 221–34
Jonsson EN, Karlsson MO. Xpose4 [online]. Available from URL: http://xpose.sourceforge.net [Accessed 2008 Mar 4]
Lindbom L, Jonsson N. PsN (Perl-speaks-NONMEM) [online]. Available from URL: http://psn.sourceforge.net [Accessed 2008 Mar 4]
Holford N. Wings for NONMEM [online]. Available from URL: http://wfn.sourceforge.net [Accessed 2008 Mar 4]
Urien S. RforNONMEM [online]. Available from URL: https://sourceforge.net/project/showfiles.php?group_id=29501 [Accessed 2008 Mar 4]
Läer S, Elshoff JP, Meibohm B, et al. Development of a safe and effective pediatric dosing regimen for sotalol based on population pharmacokinetics and pharmacodynamics in children with supraventricular tachycardia. J Am Coll Cardiol 2005; 46(7): 1322–30
Uehlinger DE, Ding RW, Schärer K. A pharmacodynamic model of erythropoietin therapy for uremic anemia. Clin Pharmacol Ther 1992; 51: 76–89
Port RE, Ding RW, Fies T, et al. Predicting the time course of haemoglobin in children treated with erythropoietin for renal anaemia. Br J Clin Pharmacol 1998; 46(5): 461–6
Jacqmin P, Snoeck E, van Schaick EA, et al. Modelling response time profiles in the absence of drug concentrations: definition and performance evaluation of the K-PD model. J Pharmacokinet Pharmacodyn 2007; 34(1): 57–85
Audren F, Tod M, Massin P, et al. Pharmacokinetic-pharmacodynamic modeling of the effect of triamcinolone acetonide on central macular thickness in patients with diabetic macular edema. Invest Ophthalmol Vis Sci 2004; 45(10): 3435–41
Pillai G, Gieschke R, Goggin T, et al. A semimechanistic and mechanistic population PK/PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br J Clin Pharmacol 2004; 58(6): 618–31
Tod M, Farcy-Afif M, Stocco J, et al. Pharmacokinetic/pharmacodynamic and time-to-event models of ribavirin-induced anaemia in chronic hepatitis C. Clin Pharmacokinet 2005; 44(4): 417–28
Gruwez B, Dauphin A, Tod M. A mathematical model for paroxetine antidepressant effect time course and its interaction with pindolol. J Pharmacokinet Pharmacodyn 2005; 32(5–6): 663–83
Gruwez B, Poirier MF, Dauphin A, et al. A kinetic-pharmacodynamic model for clinical trial simulation of antidepressant action: application to clomipraminelithium interaction. Contemp Clin Trials 2007; 28(3): 276–87
Hénin E, Zuideveld KP, Dartois C, et al. A KPD model for ordered categorical data: application to toxicity score in colorectal cancer patients treated with capecitabine [abstract no. 929; online]. Annual Meeting of the Population Approach Group in Europe; 2006 Jan 14–16; Bruges. Available from URL: http://www.page-meeting.org/?.abstract=929 [Accessed 2008 Feb 19]
Grass GM, Sinko PJ. Physiologically-based pharmacokinetic simulation modelling. Adv Drug Deliv Rev 2002; 54(3): 433–51
Nestorov I. Modelling and simulation of variability and uncertainty in toxicokinetics and pharmacokinetics. Toxicol Lett 2001; 120(1–3): 411–20
Price PS, Conolly RB, Chaisson CF, et al. Modeling interindividual variation in physiological factors used in PBPK models of humans. Crit Rev Toxicol 2003; 33: 469–503
Yang F, Tong X, McCarver DG, et al. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn 2006; 33(4): 485–518
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 2006; 95(6): 1238–57
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci 2005; 94(6): 1259–76
Nestorov IA, Aarons LJ, Arundel PA, et al. Lumping of whole-body physiologically based pharmacokinetic models. J Pharmacokinet Biopharm 1998; 26(1): 21–46
Gueorguieva I, Nestorov IA, Rowland M. Reducing whole body physiologically based pharmacokinetic models using global sensitivity analysis: diazepam case study. J Pharmacokinet Pharmacodyn 2006; 33(1): 1–27
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet 2006; 45(10): 1013–34
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet 2006; 45(9): 931–56
Wodarz D, Nowak MA. Mathematical models of HIV pathogenesis and treatment. Bioessays 2002; 24(12): 1178–87
Duval V, Chabaud S, Girard P, et al. Physiologically based model of acute ischemic stroke. J Cereb Blood Flow Metab 2002; 22(8): 1010–8
Chabaud S, Girard P, Nony P, et al. Clinical trial simulation using therapeutic effect modeling: application to ivabradine efficacy in patients with angina pectoris. J Pharmacokinet Pharmacodyn 2002; 29(4): 339–63
Post TM, Freijer JI, DeJongh J, et al. Disease system analysis: basic disease progression models in degenerative disease. Pharm Res 2005; 22(7): 1038–49
Holford NH, Chan PL, Nutt JG, et al. Disease progression and pharmacodynamics in Parkinson disease: evidence for functional protection with levodopa and other treatments. J Pharmacokinet Pharmacodyn 2006; 33(3): 281–311
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters: III. Monoexponential model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm 1983; 11(3): 303–19
Hashimoto Y, Sheiner LB. Designs for population pharmacodynamics: value of pharmacokinetic data and population analysis. J Pharmacokinet Biopharm 1991; 19(3): 333–53
Al Banna MK, Kelman AW, Whiting B. Experimental design and efficient parameter estimation in population pharmacokinetics. J Pharmacokinet Biopharm 1990; 18: 347–60
Ette EI, Howie CA, Kelman AW, et al. Experimental design and efficient parameter estimation in preclinical pharmacokinetic studies. Pharm Res 1995; 12(5): 729–37
Aarons L, Balant LP, Mentré F, et al. Practical experience and issues in designing and performing population pharmacokinetic/pharmacodynamic studies. Eur J Clin Pharmacol 1996; 49(4): 251–4
Mallet A, Mentré F. An approach to the design of experiments for estimating the distribution of parameters in random models. In: Vichinevetsky R, Borne P, Vignes J, editors. 12th IMACS World Congress; 1988 Jul 18–22. Villeneuve d’Ascq: Gerfidn, 1988: 134-7
Mentré F, Mallet A, Baccar D. Optimal design in random-effects regression models. Biometrika 1997; 84: 429–42
Merlé Y, Tod M. Impact of pharmacokinetic-pharmacodynamic model linearization on the accuracy of population information matrix and optimal design. J Pharmacokinet Biopharm 2001; 4: 365–90
Retout S, Duffull S, Mentré F. Development and implementation of the population Fisher information matrix for the evaluation of population pharmacokinetic designs. Comput Methods Programs Biomed 2001; 65(2): 141–51
Retout S, Mentré F, Bruno R. Fisher information matrix for non-linear mixedeffects models: evaluation and application for optimal design of enoxaparin population pharmacokinetics. Stat Med 2002; 21(18): 2623–39
Duffull SB, Retout S, Mentré F. The use of simulated annealing for finding optimal population designs. Comput Methods Programs Biomed 2002; 69(1): 25–35
Mentré F, Dubruc C, Thenot JP. Population pharmacokinetic analysis and optimization of the experimental design for mizolastine solution in children. J Pharmacokinet Pharmacodyn 2001; 28(3): 299–319
Duffull SB, Mentré F, Aarons L. Optimal design of a population pharmacodynamic experiment for ivabradine. Pharm Res 2001; 18(1): 83–9
Retout S, Mentré F. Optimization of individual and population designs using Splus. J Pharmacokinet Pharmacodyn 2003; 30(6): 417–43
Duffull S, Waterhouse T, Eccleston J. Some considerations on the design of population pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2005; 32(3–4): 441–57
Tod M, Mentré F, Merlé Y, et al. Robust optimal design for the estimation of hyperparameters in population pharmacokinetics. J Pharmacokinet Biopharm 1998; 26: 689–716
Dodds MG, Hooker AC, Vicini P. Robust population pharmacokinetic experiment design. J Pharmacokinet Pharmacodyn 2005; 32(1): 33–64
Foracchia M, Hooker A, Vicini P, et al. POPED, a software for optimal experiment design in population kinetics. Comput Methods Programs Biomed 2004; 74(1): 29–46
Palmer JL, Muller P. Bayesian optimal design in population models for haematologic data. Stat Med 1998; 17(14): 1613–22
Mentré F, Burtin P, Merlé Y, et al. Sparse-sampling optimal designs in pharmacokinetics and toxicokinetics. Drug Inform J 1995; 29: 997–1019
Hooker A, Vicini P. Simultaneous population optimal design for pharmacokineticpharmacodynamic experiments. AAPS J 2005 Nov 1; 7(4): E759–85
Wang J, Endrenyi L. A computationally efficient approach for the design of population pharmacokinetic studies. J Pharmacokin Biopharm 1992; 20: 279–94
Kowalski KG, Hutmacher MM. Design evaluation for a population pharmacokinetic study using clinical trial simulations: a case study. Stat Med 2001; 20(1): 75–91
Price PS, Conolly RB, Chaisson CF, et al. Modeling interindividual variation in physiological factors used in PBPK models of humans. Crit Rev Toxicol 2003; 33(5): 469–503
Nagilla R, Ward KW. A comprehensive analysis of the role of correction factors in the allometric predictivity of clearance from rat, dog, and monkey to humans. J Pharm Sci 2004; 93(10): 2522–34
West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 1999; 284(5420): 1677–9
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science 1997; 276(5309): 122–6
Anderson BJ, Allegaert K, Van den Anker JN, et al. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 2007; 63(1): 75–84
Tod M, Lokiec F, Bidault R, et al. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob Agents Chemother 2001; 45(1): 150–7
De Bony F, Tod M, Bidault R, et al. Multiple interactions of Cimetidine and probenecid with valaciclovir and its metabolite acyclovir. Antimicrob Agents Chemother 2002; 46(2): 458–63
Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci 2000; 2(1): E3
Hale M, Gillespie WR, Gupta SK, et al. Clinical trials simulation: streamlining your drug development process. Appl Clin Trial 1996; 5: 35–40
Peck CC, Barr WH, Benet LZ, et al. Opportunities for integration of pharmacokinetics, pharmacodynamics, and toxicokinetics in rational drug development. J Pharm Sci 1992; 81(6): 605–10
Holford NH, Kimko HC, Monteleone JP, et al. Simulation of clinical trials. Annu Rev Pharmacol Toxicol 2000; 40: 209–34
European Center of Pharmaceutical Medicine/Center for Drug Development Science. Frontiers in Drug Development: Computer Simulation and Modelling; 1996 Oct 18; Basel
Center for Drug Development Science. Modeling and Simulation of Clinical Trials in Drug Development and Regulation; 1997 Nov; Reston (VA)
Center for Drug Development Science (CDDS). Simulation in drug development: good practices [draft version 1.0; online]. San Francisco (CA): CDDS, 1999 Jul 23. Available from URL: http://cdds.ucsf.edu/research/sddgpreportphp [Accessed 2008 Feb 25]
Bonate PL. Clinical trial simulation in drug development. Pharm Res 2000; 17(3): 252–6
Gieschke R, Reigner BG, Steimer JL. Exploring clinical study design by computer simulation based on pharmacokinetic/pharmacodynamic modelling. Int J Clin Pharmacol Ther 1997; 35(10): 469–74
Girard P. Clinical trial simulation: a tool for understanding study failures and preventing them. Basic Clin Pharmacol Toxicol 2005; 96(3): 228–34
Girard P, Sheiner LB, Kastrissios H, et al. Do we need full compliance data for population pharmacokinetic analysis? J Pharmacokinet Biopharm 1996; 24(3): 265–82
Girard P, Blaschke TF, Kastrissios H, et al. A Markov mixed effect regression model for drug compliance. Stat Med 1998; 17(20): 2313–33
Wong D, Modi R, Ramanathan M. Assessment of Markov-dependent stochastic models for drug administration compliance. Clin Pharmacokinet 2003; 42(2): 193–204
Labbe L, Verotta D. A non-linear mixed effect dynamic model incorporating prior exposure and adherence to treatment to describe long-term therapy outcome in HIV-patients. J Pharmacokinet Pharmacodyn 2006; 33(4): 519–42
Hu C, Sale ME. A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn 2003; 30(1): 83–103
Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 1998; 64(6): 672–83
Mandema JW, Stanski DR. Population pharmacodynamic model for ketorolac analgesia. Clin Pharmacol Ther 1996; 60(6): 619–35
Veyrat-Follet C, Bruno R, Olivares R, et al. Clinical trial simulation of docetaxel in patients with cancer as a tool for dosage optimization. Clin Pharmacol Ther 2000; 68(6): 677–87
Nestorov I, Graham G, Duffull S, et al. Modeling and stimulation for clinical trial design involving a categorical response: a phase II case study with naratriptan. Pharm Res 2001; 18(8): 1210–9
Jumbe N, Yao B, Rovetti R, et al. Clinical trial simulation of a 200-microg fixed dose of darbepoetin alfa in chemotherapy-induced anemia. Oncology (Huntingt) 2002; 16 (10 Suppl.11): 37–44
Lockwood P, Ewy W, Hermann D, et al. Application of a clinical trial simulation to compare proof-of-concept study designs for drugs with a slow onset of effect; an example in Alzheimer’s disease. Pharm Res 2006; 23: 2050–9
Kimko HC, Reele SS, Holford NH, et al. Prediction of the outcome of a phase 3 clinical trial of an antischizophrenic agent (quetiapine fumarate) by simulation with a population pharmacokinetic and pharmacodynamic model. Clin Pharmacol Ther 2000; 68(5): 568–77
Krishna R, Krishnaswami S, Kittner B, et al. The utility of mixed-effects covariate analysis in rapid selection of doses in pediatric subjects: a case study with fexofenadine hydrochloride. Biopharm Drug Dispos 2004; 25(9): 373–87
Yim DS, Zhou H, Buckwalter M, et al. Population pharmacokinetic analysis and simulation of the time-concentration profile of etanercept in pediatric patients with juvenile rheumatoid arthritis. J Clin Pharmacol 2005; 45(3): 246–56
Avramis VI, Spence SA. Clinical pharmacology of asparaginases in the United States: asparaginase population pharmacokinetic and pharmacodynamic (PK/PD) models (NONMEM) in adult and pediatric ALL patients. J Pediatr Hematol Oncol 2007; 29(4): 239–47
Ramakrishnan R, Migoya E, Knorr B. A population pharmacokinetic model for montelukast disposition in adults and children. Pharm Res 2005; 22(4): 532–40
Blumer JL, Reed MD, Kaplan EL, et al. Explaining the poor bacteriologie eradication rate of single-dose ceftriaxone in group A streptococcal tonsillopharyngitis: a reverse engineering solution using pharmacodynamic modeling. Pediatrics 2005; 116(4): 927–32
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to the memory of Dr Yann Merlé, who made a substantial contribution to the work before his death in 2005.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tod, M., Jullien, V. & Pons, G. Facilitation of Drug Evaluation in Children by Population Methods and Modelling. Clin Pharmacokinet 47, 231–243 (2008). https://doi.org/10.2165/00003088-200847040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200847040-00002