Update on the management of malignant peritoneal mesothelioma
Introduction
There are approximately 800 new cases of malignant peritoneal mesothelioma (MPM) diagnosed annually in the United States with equal incidence of the disease in male and female patients (1). Several reviews helped define the natural history of the disease but did little to benefit patients with a rapidly fatal condition. Moertel (2), in his review, attributes the earliest collection of 12 documented cases of MPM to Winslow and Taylor (3). Management remained palliative until Antman et al. (4) suggested chemotherapy treatment alternatives in their management of six patients with MPM. Brenner et al. (5) defined the natural history of the disease in 25 patients as continuously localized to the peritoneal space or progressing by direct extension through the diaphragm to the pleural space. Real progress in management emerged several decades later, when a new combined surgical and regional chemotherapy treatment strategy was beginning to be popularized (6,7). The first multi-institutional consensus meeting was hosted by the National Cancer Institute in Bethesda, Maryland, in September 2004, proceedings of which were published in 2006 (8). A second international consensus statement came from the Peritoneal Surface Oncology Group Biennial Meeting in Milan, Italy, in 2006 (9). Both consensus meetings confidently proposed a combined treatment with cytoreductive surgery (CRS) followed in the operating room by hyperthermic perioperative chemotherapy (HIPEC) as the new standard of care. Emphasis on knowledgeable selection of patients at centers where the expertise of experienced caregivers is available (10-22). A multi-institutional registry evaluated CRS combined with HIPEC for MPM in 2009. These authors from 10 different institutions concluded that CRS combined with HIPEC achieved prolonged survival in selected patients with this disease (23). A meta-analysis concluded that CRS plus HIPEC has led to improved survival for patients with MPM (24). Unfortunately, substantial improvements in survival with the use of systemic chemotherapy as treatment have not been forthcoming (25-27) There have been responses showing potential palliative benefit, but survival has not been prolonged (28). The adjuvant treatment to follow CRS plus HIPEC has only recently been defined.
Unfortunately, although experienced treatment centers are able to provide optimal care for some patients with MPM in the United States and Europe, a majority of patients around the world receive only palliative care or systemic chemotherapy with cisplatin and pemetrexed or cisplatin and gemcitabine. In a review of treatment in the United States of 1,591 patients with MPM between 1973 and 2010, Miura et al. (29) concluded that approximately three of every five patients did not receive surgery when diagnosed with this disease. This failure to treat persisted despite the significant survival benefit noted in select patients. The opportunity to improve patient survival with surgical therapy was lost in a significant number of patients with MPM. The purpose of this report is to summarize recommendations for intervention with CRS accompanied by HIPEC as the first line of treatment whenever possible. This combined treatment has the potential for cure, has acceptable morbidity and mortality risks, and should become available around the globe for selected patients. It can be supplemented by adjuvant normothermic intraperitoneal chemotherapy long-term (NIPEC-LT) shown to be successful in single institution studies. A challenge is to provide knowledgeable and technically proficient management worldwide.
Diagnosis and patient selection
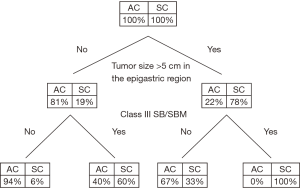
The most common symptoms of MPM are pain (dry type) and/or ascites (wet type). Patients often require a biopsy, usually using computed tomography (CT) guidance or laparoscopy through the linea alba (15). Surprisingly, peritoneal cytology of ascites often does not establish a definitive diagnosis. Pathology must be reviewed. A core or tissue biopsy is preferred. In most cases, histopathology of the tumor shows epithelioid features, although it is difficult to predict biologic behavior (aggressive vs. indolent) based on histopathology alone. Sarcomatoid or biphasic histologic types are usually excluded from CRS and HIPEC (Figure 1). CT of chest, abdomen, and pelvis or CT of chest plus magnetic resonance imaging of abdomen and pelvis is obtained. An experienced radiologist must communicate the presence or lack of so-called concerning radiologic features. This radiologic interpretation should contribute to the judgments regarding probability of complete cytoreduction versus debulking surgery (30-32) (Figure 2). A consensus regarding criteria for patient selection for potentially curative treatment is provided in Table 1 which lists favorable and guarded clinical features.
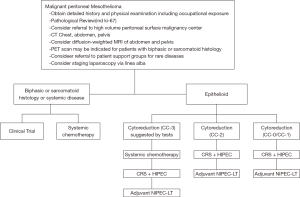

Full table
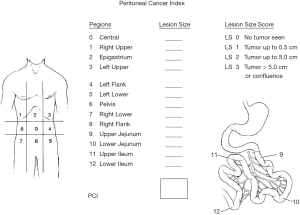
The selection of patients must be based on host and disease factors. The extent of disease profoundly influences the completeness of cytoreduction. This important assessment is best estimated using an intraoperative quantitation of all sites of disease within the abdomen and pelvis, known as the peritoneal cancer index (Figure 3). The distribution and extent of disease at 13 specific sites are recorded at the time of exploration as part of the patient’s permanent record (33).
The safety and feasibility of laparoscopy have been demonstrated in patients with peritoneal surface malignancies by several groups (34,35). Although laparoscopy may lead to understaging of the disease burden, it is a useful technique to determine if some patients have extensive burden of disease, which may preclude the benefit of cytoreduction. Laparoscopy port site recurrence is a consideration that must be noted in placement of ports (Figure 4). Laparoscopy ports must be limited to the midline so that port sites developing progressive disease can be excised in the absence of excessive surgical trauma to the abdominal wall (36).
CRS
If a patient does not have coexisting medical conditions that would result in high surgical risk and if complete cytoreduction or significant debulking is predicted, CRS should be performed. Surgery must be performed by an experienced surgical team, with the understanding that complete cytoreduction may require up to six peritonectomy procedures and multiple visceral resections (7). The surgical team must have experience in making the necessary intraoperative judgments regarding the different surgical maneuvers that may be needed and must be proficient with these procedures. Although the extent of peritonectomy is limited at most institutions to peritoneal surfaces visibly infiltrated by disease (selective peritonectomy), other groups have recommended total parietal peritonectomy (systematic peritonectomy) (37). Mesenteric peritonectomy has been added as a peritonectomy procedure to increase the proportion of patients with a complete cytoreduction (38).
Presence of lymph node metastases has been shown to be a factor associated with shortened survival, and lymph node metastases are included in a proposed staging system for patients with MPM (39,40). During the CRS procedure, assessment of the lymph nodes in the regions that are explored surgically should be routinely performed by all centers. All enlarged lymph nodes should be removed and submitted for permanent histologic section. Node sampling is also recommended. Lymph node groups that have been recommended for histopathologic assessment to rule out lymph node positivity include the deep epigastric lymph nodes, external iliac lymph nodes at the internal inguinal ring, common iliac lymph nodes, or accessible lymph nodes present in the mediastinum immediately above the superior surface of the diaphragm, especially if these lymph nodes are enlarged by pre-treatment CT.
Although there is complete agreement regarding the importance of complete or near-complete cytoreduction in the surgical management of MPM, some controversy does exist regarding the use of selective versus complete parietal peritonectomy. Baratti et al. (37) reported a 5-year survival rate with selective peritonectomy of 40%, as compared with a survival rate of 63.9% with complete parietal peritonectomy (P=5.0269). They performed this complete parietal peritonectomy without increased morbidity or mortality.
MPM has a pattern of intraperitoneal dissemination considerably different from that of other malignancies with metastases to peritoneal surfaces. The redistribution characteristic of mucinous appendiceal tumor with relative sparing of small bowel and its mesentery is rarely observed (39). Parietal peritoneal surfaces are typically diffusely involved, and extensive peritonectomy is usually required. The perihepatic regions may present a considerable challenge, especially the posterior aspect of the hepatoduodenal ligament. A unique finding in MPM is extensive involvement of small- and large-bowel mesenteries, with sparing of the surface of the bowel. In some patients, to achieve a complete cytoreduction, an attempt to remove the visceral peritoneum on the small bowel mesentery must be made (38,40). Lymph nodes are removed selectively if mesothelioma infiltration is suspected (41,42). Complete cytoreduction may require a combination of small- and large-bowel resections, especially of splenic flexure and rectosigmoid colon.
Hyperthermic perioperative chemotherapy
Immediately after CRS and before intestinal reconstruction and abdominal closure, the abdomen and pelvis must be prepared for HIPEC. Hemostasis must be complete, or bleeding during HIPEC will occur. Extensive irrigation is indicated to mechanically clear loose cancer cells from all peritoneal surfaces. Extensive intraoperative peritoneal lavage has been used with good results in gastric cancer (43). Some have recommended copious distilled water, whereas other groups have used diluted (0.25%) hydrogen peroxide or povidone iodine (44,45).
After mechanical cleansing of the peritoneal space by irrigation, all patients who undergo complete or near-complete cytoreduction should be treated with HIPEC. Standard recommendations include the use of a platinum-based agent such as cisplatin if renal function is adequate (24). Different chemotherapeutic options have been explored, including high-dose cisplatin (250 mg/m2), cisplatin plus doxorubicin, cisplatin plus mitomycin, and mitomycin alone (Table 2). One option is to use bidirectional chemotherapy by adding systemic ifosfamide plus mesna disulfide by continuous infusion for the 90 minutes of HIPEC with doxorubicin and cisplatin (46). Two retrospective studies have shown an association with better survival using cisplatin compared with mitomycin (22,24). In the absence of data derived from prospectively conducted clinical trials, an HIPEC regimen familiar to the caregivers must be considered an institutional standard of care for patients with MPM.
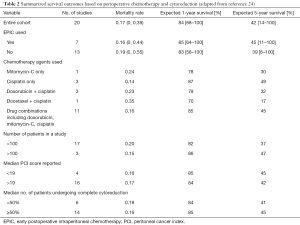
Full table
NIPEC-LT
An additional treatment currently routinely used at some institutions is long-term intraperitoneal chemotherapy through an intraperitoneal port combined with systemic chemotherapy (47). These bidirectional intraperitoneal/systemic chemotherapy regimens are similar to those successfully used for ovarian cancer (48). Sugarbaker and Chang compared the outcome in consecutive patients who received CRS plus HIPEC to patients who received CRS plus HIPEC plus NIPEC-LT. The adjuvant treatment was delivered through an intraperitoneal port placed at the time of CRS. Pemetrexed at 500 mg/m2 intraperitoneal was combined with cisplatin 75 mg/m2 intravenously every 3 weeks for 6 cycles. This non-randomized trial showed a statistically beneficial effect with a P value of 0.01. An updated survival of CRS plus HIPEC ± NIPEC-LT is shown in Figure 5 (47).
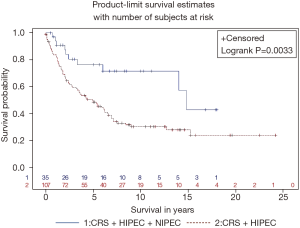
There is a new concept regarding the use of HIPEC in MPM after acceptable (CC-0/CC-1/CC-2) cytoreduction. HIPEC is a necessary part of the management plan but is not sufficient in these patients who are at high risk of local-regional recurrence. The roles of other local-regional chemotherapy treatment strategies such as NIPEC-LT are being evaluated in clinical studies.
Palliation
In some patients, clinical and radiologic evaluation indicates a small likelihood of complete or near-complete cytoreduction. Current data would recommend a palliative surgical intervention in selected patients (49). If significant debulking can be achieved, HIPEC is an optional treatment and may be recommended if ascites is a prominent part of the patient’s symptoms. HIPEC, even in the absence of therapeutic CRS, has been associated with durable control of symptomatic malignant ascites in a majority of patients (20).
Open versus closed HIPEC
Although the technology for CRS for MPM is uniform at a majority of peritoneal surface malignancy treatment centers, there remains considerable contrast regarding the methodology for delivery of HIPEC (50). Some centers use a closed technique, where the intestinal anastomoses and the abdominal incision are completed before initiation of HIPEC. Other centers concerned about the uniformity of distribution of the heat and chemotherapy solution advocate an open technique, with manual distribution of the chemotherapy solution. At this point in time, no difference in survival between the two techniques has been published. Also, because each institution tends to follow its own methodology in all patients, comparisons of the safety of the open versus closed technique from a patient perspective are not available (51). To date, no group studying the open technique has identified potentially environmentally dangerous aerosols.
Contrast of CRS for MPM versus gastrointestinal cancer
There are differences in the selection factors for CRS plus HIPEC in patients with MPM as compared with patients with peritoneal metastases from gastrointestinal cancer. Data would suggest that patients with MPM with significant debulking yet incomplete cytoreduction, significantly benefit from the use of HIPEC. In other words, if CRS can be used to separate all of the bowel loops and reduce the size of the nodules to 1 cm or smaller, HIPEC is considered to be of benefit and, in some instances, is associated with long-term progression-free survival. Sugarbaker et al. (15) found a clear difference in patient survival when CC-0, CC-1, and CC-2 cytoreductions were compared with CC-3 cytoreductions. However, no statistically significant difference was seen when CC-0 and CC-1 cytoreductions were compared with CC-2 cytoreductions. Accepting the fact that CC-0 to CC-1 cytoreduction is always the goal, an incomplete cytoreduction plus HIPEC is a rationale management plan in MPM (29). There is a clear contrast in the management plan of patients who undergo complete versus incomplete cytoreduction for high-grade gastrointestinal cancer. As reported by Goéré et al. (52) in performing abdominal exploration for a gastrointestinal malignancy with peritoneal metastases, if complete cytoreduction is judged to be impossible, the procedure is either aborted or palliative intervention becomes the goal of surgery in the absence of HIPEC.
Morbidity and mortality of CRS and HIPEC
For CRS and HIPEC to be regarded as standard of care in selected patients, the morbidity and mortality risks must be acceptable. This combined treatment involves a long surgical procedure (up to 12 hours) with perioperative intraperitoneal chemotherapy. There is potential for intraoperative and/or postoperative complications, even postoperative death.
Although experienced centers have reported an acceptable incidence of mortality and morbidity, a steep learning curve for this combined treatment exists and presents a major challenge in educational efforts. Although data were not specific for MPM, Kusamura et al. (53) showed that transition from suboptimal performance to technologic and oncologic success required a median of 100 procedures. Outcomes at centers early in their experience may be considerably less optimal than those at experienced centers. To qualify as an experienced center with more than 50 patients per year, not all patient cases must involve MPM. However, this diagnosis includes peritoneal metastases patients who will require a team effort with maximal knowledge and technical expertise for an optimal outcome.
In this last decade, several experienced peritoneal surface malignancy centers have reported adverse events, including operative mortality for MPM patients treated with CRS plus HIPEC. Yan et al. (54) in 2007 reported on 70 consecutive patients; the mortality rate was 3%. Rates of grade 3 and 4 adverse events were 27% and 14%, respectively. Primary colonic anastomosis (P=5.028), more than four peritonectomy procedures (P=5.015), and duration of surgery of more than 7 hours (P=5.027) were the risk factors for grade 4 morbidity. Yano et al. (20) in 2008 reported on 17 patients who underwent 18 laparotomies. Seven (41%) developed complications, and one patient died postoperatively. The most common complications were pneumonia (12%) and prolonged ileus (12%). Chua et al. (55) in 2009 reported on 20 patients treated with CRS plus HIPEC for MPM. Three patients experienced grade 3 postoperative complications: two had pneumothorax, and one had pleural effusion. One patient with grade 4 complications had a bile leak causing peritonitis. One patient died in the intensive care unit after a 14-day stay from sepsis and multiorgan failure.
Yan et al. (23) collected data on 401 patients from eight institutions. All but 21 patients underwent the surgical intervention plus perioperative chemotherapy. Eleven (3%) experienced cardiac complications; 46 (11%) experienced respiratory complications; 74 (18%) experienced bowel related adverse events; 39 (10%) experienced renal complications; 25 (6%) developed hematologic toxicity. Overall, 127 patients (31%) experienced grade 3 or 4 complications. Nine patients (2%) died perioperatively. Mean length of hospital stay was 22 days (standard deviation, 15 days).
Repeat cytoreduction of limited recurrence diagnosed in follow-up
Two recent reports indicated that the follow-up of patients treated with CRS plus HIPEC for MPM must be thorough and prolonged. Wong et al. (56) treated 29 patients for MPM, and eight underwent additional repeat HIPEC. Complications occurred in 65% of the patients treated with single HIPEC and 50% of patients who underwent repeat HIPEC. Patients who underwent repeat HIPEC had improved median survival versus those undergoing single HIPEC (P=5.007).
Ihemelandu et al. (57) studied 205 consecutive patients treated with CRS plus HIPEC; 44 (21.5%) underwent repeat CRS and HIPEC for progressive disease. There was no 30-day mortality, and the grade 4 morbidity rate was 2%. The 5-year survival rate for patients undergoing repeat CRS and HIPEC was 46% versus 52% for those undergoing a single intervention with CRS and HIPEC. The authors concluded that patients undergoing CRS and HIPEC for MPM should be observed carefully to see if repeat CC-0 or CC-1 cytoreduction plus HIPEC was possible when disease progressed. In conclusion, management of MPM requires careful selection of patients and appropriate use of CRS and HIPEC for patients suitable for this treatment. Complete or near-complete cytoreduction and use of platinum-based HIPEC are essential to optimize the possibility of long-term survival in these patients.
Summary
The natural history of MPM is defined by progression restricted to the peritoneal space. In the past, patients with this disease had a limited survival of approximately 1 year. Numerous studies have reported median survival of 3 to 5 years with a combination of CRS and HIPEC. These markedly improved survival statistics were achieved in experienced centers with a 1% mortality and a 20% morbidity. Knowledgeable patient selection is required to prevent patients unlikely to profit from undergoing extensive procedures. Patients with NIPEC-LT have improved survival when this intervention is added to CRS plus HIPEC. This management plan may be the standard of care for properly selected patients with MPM patients at experienced centers.
Acknowledgements
Manuscript preparation costs provided by the Foundation for Applied Research in Gastrointestinal Oncology (FARGO).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control 2009;20:935-44. [Crossref] [PubMed]
- Moertel CG. Peritoneal mesothelioma. Gastroenterology 1972;63:346-50. [PubMed]
- WINSLOW DJ. TAYLOR HB. Malignant peritoneal mesotheliomas: a clinicopathological analysis of 12 fatal cases. Cancer 1960;13:127-36. [Crossref] [PubMed]
- Antman KH, Blum RH, Greenberger JS, et al. Multimodality therapy for malignant mesothelioma based on a study of natural history. Am J Med 1980;68:356-62. [Crossref] [PubMed]
- Brenner J, Sordillo PP, Magill GB, et al. Malignant peritoneal mesothelioma: review of 25 patients. Am J Gastroenterol 1981;75:311-3. [PubMed]
- Dedrick RL. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin Oncol 1985;12:1-6. [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Hassan R, Alexander R, Antman K, et al. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol 2006;17:1615-9. [Crossref] [PubMed]
- Deraco M, Bartlett D, Kusamura S, et al. Consensus statement on peritoneal mesothelioma. J Surg Oncol 2008;98:268-72. [Crossref] [PubMed]
- Averbach AM, Sugarbaker PH. Peritoneal mesothelioma: Treatment approach based on natural history. In: Sugarbaker PH. Editor. Peritoneal Carcinomatosis: Drugs and Diseases. Boston: Kluwer, 1996:193-211.
- Ma GY, Bartlett DL, Reed E, et al. Continuous hyperthermic peritoneal perfusion with cisplatin for the treatment of peritoneal mesothelioma. Cancer J Sci Am 1997;3:174-9. [PubMed]
- Park BJ, Alexander HR, Libutti SK, et al. Treatment of primary peritoneal mesothelioma by continuous hyperthermic peritoneal perfusion (CHPP). Ann Surg Oncol 1999;6:582-90. [Crossref] [PubMed]
- Mongero LB, Beck JR, Kroslowitz RM, et al. Treatment of primary peritoneal mesothelioma by hyperthemic intraperitoneal chemotherapy. Perfusion 1999;14:141-5. [Crossref] [PubMed]
- Loggie BW, Fleming RA, McQuellon RP, et al. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg 2001;67:999-1003. [PubMed]
- Sugarbaker PH, Welch LS, Mohamed F, et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605-21. xi. [Crossref] [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [Crossref] [PubMed]
- Nonaka D, Kusamura S, Baratti D, et al. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated locoregionally at a single institution. Cancer 2005;104:2181-8. [Crossref] [PubMed]
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol 2006;13:405-12. [Crossref] [PubMed]
- Elias D, Bedard V, Bouzid T, et al. Malignant peritoneal mesothelioma: treatment with maximal cytoreductive surgery plus intraperitoneal chemotherapy. Gastroenterol Clin Biol 2007;31:784-8. [Crossref] [PubMed]
- Yano H, Moran BJ, Cecil TD, et al. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur J Surg Oncol 2009;35:980-5. [Crossref] [PubMed]
- Chua TC, Chu F, Morris DL. Outcomes of single-centre experience of hepatic resection and cryoablation of sarcoma liver metastases. Am J Clin Oncol 2011;34:317-20. [Crossref] [PubMed]
- Blackham AU, Shen P, Stewart JH, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol 2010;17:2720-7. Erratum in: Ann Surg Oncol 2011;18 Suppl 3:S325-6. [Crossref] [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [Crossref] [PubMed]
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686-93. [Crossref] [PubMed]
- Garcia-Carbonero R, Paz-Ares L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur J Surg Oncol 2006;32:676-81. [Crossref] [PubMed]
- Jänne PA, Wozniak AJ, Belani CP, et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer 2005;7:40-6. [Crossref] [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [Crossref] [PubMed]
- Lainakis G, Zagouri F, Kastritis E, et al. Systemic chemotherapy with pemetrexed and cisplatin for malignant peritoneal mesothelioma: a single institution experience. Tumori 2011;97:25-9. [Crossref] [PubMed]
- Miura JT, Johnston FM, Gamblin TC, et al. Current trends in the management of malignant peritoneal mesothelioma. Ann Surg Oncol 2014;21:3947-53. [Crossref] [PubMed]
- Yan TD, Haveric N, Carmignani CP, et al. Computed tomographic characterization of malignant peritoneal mesothelioma. Tumori 2005;91:394-400. [Crossref] [PubMed]
- Yan TD, Haveric N, Carmignani CP, et al. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer 2005;103:839-49. [Crossref] [PubMed]
- Sugarbaker PH, Sardi A, Brown G, et al. Concerning CT features used to select patients for treatment of peritoneal metastases, a pictoral essay. Int J Hyperthermia 2017;33:497-504. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 1996;15:49-58.
- Valle M, Federici O, Garofalo A. Patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, and role of laparoscopy in diagnosis, staging, and treatment. Surg Oncol Clin N Am 2012;21:515-31. [Crossref] [PubMed]
- Tabrizian P, Jayakrishnan TT, Zacharias A, et al. Incorporation of diagnostic laparoscopy in the management algorithm for patients with peritoneal metastases: A multi-institutional analysis. J Surg Oncol 2015;111:1035-40. [Crossref] [PubMed]
- Muensterer OJ, Averbach AM, Jacquet P, et al. Malignant peritoneal mesothelioma. Case-report demonstrating pitfalls of diagnostic laparoscopy. Int Surg 1997;82:240-3. [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol 2012;19:1416-24. [Crossref] [PubMed]
- Deraco M, Baratti D, Kusamura S, et al. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J Surg Oncol 2009;100:321-8. [Crossref] [PubMed]
- Carmignani CP, Sugarbaker TA, Bromley CM, et al. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev 2003;22:465-72. [Crossref] [PubMed]
- Deraco M, Baratti D, Kusamura S. Diffuse malignant peritoneal mesothelioma. In: Sugarbaker PH. Editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. Woodbury, CT: Cine-Med Publishing, 2012:115-26.
- Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 2006;32:948-53. [Crossref] [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Lymph node metastases in diffuse malignant peritoneal mesothelioma. Ann Surg Oncol 2010;17:45-53. [Crossref] [PubMed]
- Shimada S, Kuramoto M, Marutsuka T, et al. Adopting extensive intra-operative peritoneal lavage (EIPL) as the standard prophylactic strategy for peritoneal recurrence. Rev Recent Clin Trials 2011;6:266-70. [Crossref] [PubMed]
- Harrison LE, Tiesi G, Razavi R, et al. A phase I trial of thermal sensitization using induced oxidative stress in the context of HIPEC. Ann Surg Oncol 2013;20:1843-50. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [Crossref] [PubMed]
- Van der Speeten K, Stuart OA, Sugarbaker PH. Cancer chemotherapy for peritoneal metastases: Pharmacology and treatment. In: Sugarbaker PH. Editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd Edition. Woodbury, CT: Cine-Med Publishing, 2017:47-82.
- Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol 2017;43:1228-35. [Crossref] [PubMed]
- Landrum LM, Java J, Mathews CA, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol 2013;130:12-8. [Crossref] [PubMed]
- Rodríguez D, Cheung MC, Housri N, et al. Malignant abdominal mesothelioma: defining the role of surgery. J Surg Oncol 2009;99:51-7. [Crossref] [PubMed]
- Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol 2016;7:29-44. [PubMed]
- Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol 2008;98:242-6. [Crossref] [PubMed]
- Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 2015;22:2958-64. [Crossref] [PubMed]
- Kusamura S, Moran BJ, Sugarbaker PH, et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br J Surg 2014;101:1758-65. [Crossref] [PubMed]
- Yan TD, Edwards G, Alderman R, et al. Morbidity and mortality assessment of cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma--a prospective study of 70 consecutive cases. Ann Surg Oncol 2007;14:515-25. [Crossref] [PubMed]
- Chua TC, Yan TD, Morris DL. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: the Australian experience. J Surg Oncol 2009;99:109-13. [Crossref] [PubMed]
- Wong J, Koch AL, Deneve JL, et al. Repeat cytoreductive surgery and heated intraperitoneal chemotherapy may offer survival benefit for intraperitoneal mesothelioma: a single institution experience. Ann Surg Oncol 2014;21:1480-6. [Crossref] [PubMed]
- Ihemelandu C, Bijelic L, Sugarbaker PH. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent or progressive diffuse malignant peritoneal mesothelioma: clinicopathologic characteristics and survival outcome. Ann Surg Oncol 2015;22:1680-5. [Crossref] [PubMed]


