Association of circulating total bilirubin level with ischemic stroke: a systemic review and meta-analysis of observational evidence
Introduction
Stroke accounts for about 1/3 of causes of death worldwide (1). Of great importance, stroke has been the leading cause of death in China in recent years (2). Although much progress has been made in identifying risk factors for stroke, little is known about factors that modulate stroke risk. Serum bilirubin is a normal metabolite of the heme. Traditionally, bilirubin is regarded as a toxic metabolite. In clinical practice, serum bilirubin has been used a marker of liver and hematopoietic diseases. In recent years, a variety of studies have indicated that bilirubin may exert anti-oxidative, anti-inflammatory and neuroprotective effects (3). Some studies have revealed that circulating total bilirubin is negatively related to the risk for ischemic stroke (4-6). Perlstein et al. conducted a cross-sectional study on 13,214 adult participants in whom 453 reported a history of stroke; their results showed an increment of total bilirubin by 1.71 µmol/L could reduce the incidence of ischemic stroke by 9% (7). However, there is still controversy on this issue. Kurzepa et al. found serum total bilirubin had a weak relationship with the risk for ischemic stroke (8). Kunutsor et al. investigated the correlation of circulating total bilirubin with the risk of incident cardiovascular disease in 12 prospective studies involving 9,378 subjects by meta-analysis, but the stroke was not used as an endpoint (9). Li et al. investigated the serum total bilirubin in a Chinese population with acute stroke as an endpoint, but only case-control studies were included (10).
Considering that serum total bilirubin is an important biomarker of stroke and has a potential value in the prediction of outcome of stroke, it is imperative to systemically evaluate the correlation between circulating total bilirubin and risk for stroke. This systemic review and meta-analysis was conducted to investigate the relationship between circulating total bilirubin and risk for ischemic stroke.
Methods
Literature search
This study was registered at PROSPERO [https://www.crd.york.ac.uk/PROSPERO/(CRD42017075988)] and reported in accordance with the PRISMA (11) and MOOSE (12) guidelines. PubMed, EMBASE, Web of Science and Cochrane Central were searched for the relevant studies published before 30 June 2017. Studies that examined the association between circulating bilirubin level and stroke in adults (≥18 years) were included for further analysis. Terms related to bilirubin (such as “hyperbilirubin”) were combined with key terms related to the outcome (such as “stroke”); no language restriction was applied. The exact search strategy and rationale are shown in the Supplementary file 1.
Study selection and inclusion criteria
Cohort, case-control and cross-sectional studies that examined the association between blood bilirubin level (total bilirubin, direct or indirect bilirubin levels) and stroke were included for further analysis. Two reviewers (P Zhong and D Wu) independently reviewed the title, abstract and full-text, and then input data into a data extraction form. A third reviewer (X Wang) approved the studies selected. When the data were missing, the principal investigators were contacted for further information. If the principal investigator could not provide the missing data, the study was excluded. Finally, the full texts of included studies were retrieved. The primary outcome of this study was ischemic stroke, and the secondary outcome was stroke.
Data extraction
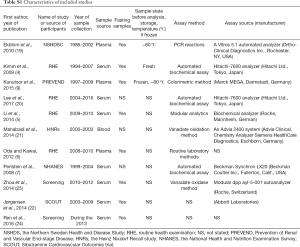
Two investigators independently extract the data and filled them in the form above. Following information was recorded: the first author, publication date, study design, study name, geographical location, population source, time of baseline survey, sample population, sample source (serum/plasma), sample nature (fresh or frozen and storage temperature), assay type, case definition, sample size, sex, mean age at baseline, number of outcome events, summary statistics (using a standardized abstraction form) and degree of adjustment for potential confounders (Table S1).
Full table
Adjustments were classified as: “+” when risk estimates were adjusted for age and sex; “++” when it was further adjusted for potential risk factors [such as blood pressure, body mass index (BMI)], history of diabetes mellitus, smoking, drinking, excise status and medication); and “+++” when it was further adjusted for inflammatory markers [such as C-reactive protein (CRP)], liver enzymes and blood lipids. The estimates reported with the greatest degree of adjustment were also recorded. If risk estimates were not available, the authors were contacted for further information (Table S1).
Quality assessment
Study quality was evaluated using the Newcastle-Ottawa Scale (NOS) for cohort studies and case-control studies (13). The quality of studies was determined according to the selection criteria for participants, comparability of cases and controls, and exposure and outcome assessments. For cross-sectional studies, quality was assessed using the NOS modified for cross-sectional studies (14). Over all, a score ≥5 indicated adequate quality for inclusion in the present review (Tables S2,S3).

Full table
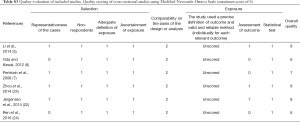
Full table
Statistical analysis
The effect of bilirubin levels on the risk for stroke was evaluated on the basis of included studies. Odds ratio (OR) was used for the evaluation of the relationship between bilirubin levels and stroke. Because different results were reported in the studies [OR per standard deviation (SD) and OR per quartile], the results were first transformed into OR between upper tertile to lower tertile (15,16), and then the differences observed between groups were expressed as OR with corresponding 95% confidence interval (CI). Individual adjusted OR and 95% CI were extracted or calculated first, and then log OR and its corresponding standard error were estimated for pooling. The I2 statistic and χ2 test were employed to assess the variability across studies attributable to heterogeneity beyond chance. A P value greater than 0.10 in the χ2 test was interpreted as low-level heterogeneity (17). A pooled effect was calculated with a fixed-effects model when there was no statistically significant heterogeneity; otherwise, a random effects model was employed. Subgroup was used to determine the robustness across different groups. Publication bias was evaluated by Egger test and funnel plot. Trim and fill method was used for adjustment once bias was present (18). A value of two-sided P value less than 0.05 was considered statistically significant. Statistical analysis was conducted with the Review Manager (version 5.1 for Windows, Cochrane Collaboration, Oxford, UK, 2011) and STATA 11.0 (Stata Corporation, Lakeway, Texas, USA).
Results
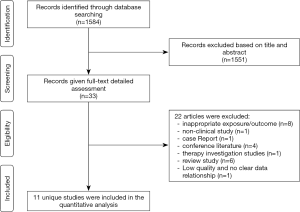
A total of 1,584 studies were identified after initial searching. After reviewing the title and abstract, the full texts of 33 studies were obtained, in which 11 studies were included for further analysis and 22 studies were excluded because they did not meet the inclusion criteria (Figure 1). In 11 studies, there were five prospective studies (4,9,19-21) [1 with Mendelian randomization (MR) (16)] and six cross-sectional studies (5,6,22-25). Of these studies, there were 131,450 subjects, including 58,168 females (44.3%). The general features of included studies are shown in Table 1. In the included studies, the serum bilirubin was detected in a fasting condition (Table S1).
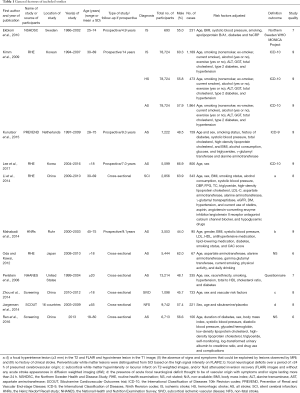
Full table
Correlation between bilirubin level and ischemic stroke
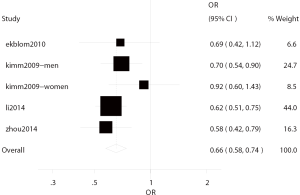
Four studies (4,5,19,25) investigated the correlation between bilirubin level and ischemic stroke, including two cross-sectional studies (5,25) and two prospective studies (4,19) with involvement of 83,380 subjects. In these studies, ischemic stroke was found in 2,496 patients from Korea, China and Sweden, and the median age was 56.56 years. There was no heterogeneity among four studies (I2=0.0%, P=0.451), and thus fixed effects model was used for further analysis. In four studies, the total OR of the highest bilirubin level and the lowest bilirubin level for ischemic stroke was 0.66 (95% CI: 0.58–0.74) (Figure 2).
In two prospective studies (4,19), adjustment was done for the potential risk factors and the total OR of the ratio of highest bilirubin level to lowest bilirubin level for ischemic stroke was 0.74 (95% CI: 0.61–0.91), showing significant difference. In the two cross-sectional studies (5,25), after adjustment for potential risk factors, the total OR of the ratio of highest bilirubin level to lowest bilirubin level for ischemic stroke was 0.61 (95% CI: 0.51–0.71), showing significant difference. There was no heterogeneity in the prospective studies (I2=0.0%, P=0.540) and cross-sectional studies (I2=0.0%, P=0.740).
Stratified analysis was done according to the gender. The OR was 0.72 for males (95% CI: 0.57–0.91) with significant difference and 0.78 for females (95% CI: 0.53–1.16) without significant difference. In addition, significant difference was not observed between patients aged ≥60 years and those younger than 60 years; subgroup analysis of studies with involvement of ≥2,000 subjects and those with no more than 2,000 subjects also showed no significant difference (Table S4).
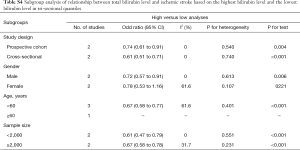
Full table
Correlation of bilirubin level with stroke
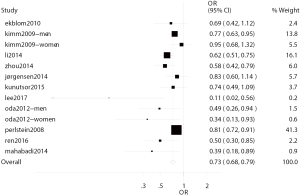
In 11 studies, the correlation between bilirubin level and stroke was investigated (4-7,9,19-22,24,25). There were six cross-sectional studies (5-7,22,24,25) and five prospective studies (4,9,19-21) with involvement of 131,450 subjects. In these studies, stroke was reported in 5,060 patients, and the subjects in these studies were from China, Japan, Korea, Sweden, Netherlands, Germany, and USA. There was heterogeneity among 11 studies (I2=51.4%, P=0.016), and thus random effects model was used (Figure 3).
In six cross-sectional studies (5,6,22-25), the total OR of the ratio of highest bilirubin level to lowest bilirubin level for stroke was 0.65 (95% CI: 0.54–0.79) after adjustment for potential risk factors, showing significant difference. In addition, in five prospective studies (4,9,19-21), the total RR of the ratio of highest bilirubin level to lowest bilirubin level for stroke was 0.72 (95% CI: 0.56–0.92) after adjustment for potential risk factors, showing significant difference. Heterogeneity was noted in six cross-sectional studies (I2=59.3%, P=0.022) and prospective studies (I2=48.0%, P=0.087).
Stratified analysis based on the sex showed the OR was 0.75 (95% CI: 0.62–0.90) for males and 0.57 (95% CI: 0.28–1.17) for females, showing no marked differences (Table S5). In addition, significant difference was not observed between patients aged ≥60 years and those younger than 60 years; subgroup analysis of studies with involvement of ≥2,000 subjects and those with no more than 2,000 subjects also showed no significant difference (Table S5).
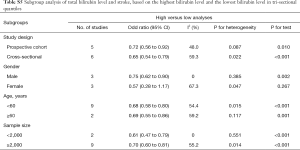
Full table
Assessment and management of risk for bias
Results of the Egger test for ischemic stroke (P=0.317) suggested that any publication bias across included studies was unlikely (Figure S1). Results of the Egger test for stroke (P=0.011) suggested that any publication bias across included studies was likely (Figure S1). Trim and fill method was used for the adjustment. After adjustment, the total OR of ratio of highest bilirubin level to lowest bilirubin level for stroke/ischemic stroke was 0.638 (95% CI: 0.565–0.721; P≤0.0001), showing significant difference.

Discussion
This meta-analysis was conducted to systemically investigate the relationship between circulating total bilirubin level and risk for ischemic stroke and stroke on the basis of available observational studies. The cross-sectional studies and prospective studies indicated that the serum bilirubin level was negatively related to the risk for ischemic stroke and stroke after complete adjustment.
The negative relationship between circulating total bilirubin level and risk for ischemic stroke/stroke was consistent with previously reported. Studies have revealed that bilirubin level may serve as a predictor of some vascular events such as coronary heart disease (26), peripheral artery disease (23), amputation of diabetes mellitus patients (27), diabetic nephropathy (28) and overall mortality (29). Gilbert’s syndrome (GS) is a relatively common condition, inducing a benign, non-hemolytic, and unconjugated hyperbilirubinemia. GS patients often present mildly elevated plasma anti-oxidative capacity due to the elevation of unconjugated bilirubin (UCB) and reductions in the thiols and glutathione. Interestingly, the incidence of cardiovascular disease and risk for death from cardiovascular diseases are remarkably reduced in GS patients (30), which might be explained by anti-oxidative capability of bilirubin according to available findings. Baranano et al. (31) proposed a mechanism for the cytoprotective action of bilirubin based on an amplification cycle whereby the bile pigment, in the presence of albumin, is itself oxidized to biliverdin by reactive oxygen species (ROS) and, then, recycled by biliverdin reductase back to bilirubin. This hypothesis was challenged by Maghzal et al. (32) and McDonagh (33) who demonstrated that the oxidation of both bilirubin and albumin-bound bilirubin by peroxyl radicals or hydrogen peroxide largely degrades bilirubin and generates only negligible amounts of biliverdin.
Stroke can be divided into ischemic stroke and hemorrhagic stroke. The present meta-analysis showed circulating total bilirubin level was negatively related to the incidence of stroke. In the included studies, only one focused on the relationship between total bilirubin level and hemorrhagic stroke, while results showed no significant relationship between them. Among all included studies, only one study focused on relationship between total bilirubin level and hemorrhagic stroke, while results showed no significant relationship between them (34). Studies on the correlation between bilirubin and hemorrhagic stroke mainly focus on the relationship of bilirubin level with the prognosis of hemorrhagic stroke. Dohi et al. (35) found the serum bilirubin increased significantly in the early phase of cerebral hemorrhage and subarachnoid hemorrhage and they speculated that serum bilirubin concentration might serve as a useful marker of oxidative stress in hemorrhagic stroke patients. However, there is evidence showing that a large amount of unbound bilirubin is produced after cerebral hemorrhage, which may cause serious damage to the brain and induce brain edema (36), leading to the deterioration of cerebral hemorrhage. Among included studies, clinical types of ischemic and hemorrhagic stroke were not classified in six studies. The pathophysiology of ischemic stroke is different from that of hemorrhagic stroke, and thus it is necessary to investigate the correlation between serum bilirubin level and stroke of different types. Moreover, ischemic stroke can also be divided into different clinical subtypes. Lin et al. investigated 628 patients with ischemic stroke according to the clinical characteristics and imaging findings (37). In this study, etiological grouping was done according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST), and their results showed the increase in the serum bilirubin level was an independent predictor of cardiogenic brain embolism. In the included studies, Li et al. conducted a cross-sectional study with involvement of 2,865 subjects. Their results showed the total bilirubin level in the silent cerebral infarction (SCI) group was significantly lower than in the non-SCI group, and multivariate analysis indicated high total bilirubin level was independently related to the reduced risk for SCI (OR 0.925; 95% CI: 0.897–0.954; P<0.001). This indicates that total bilirubin level can be used as a new biomarker of asymptomatic cerebral infarction (5). More studies are needed to confirm the relationship between serum total bilirubin level and risk for stroke of different types.
Our meta-analysis showed the relationship between serum total bilirubin level and incidence of ischemic stroke/stroke was statistically significant in males, but not in females. Kimm et al. investigated 78,724 healthy subjects in a prospective study with the median duration of follow-up of 9.4 years. Their results showed patients with high serum bilirubin level had a reduced incidence of ischemic stroke as compared to those with low serum bilirubin level, and the negative relationship between serum bilirubin level and ischemic stroke was only noted in males, not in females (4). In the whole population, the serum bilirubin level in males was slightly higher than in females (23). The difference in serum bilirubin between females and males might be ascribed to the differences in serum estrogen, iron storage in males, higher heme oxygenase in males and lifestyle [drinking, smoking and supplement of anti-oxidants (such as vitamin C)] between them (38,39). Clinical and experimental studies indicated significant differences of liver function between different genders when circulation was poor (39). Under circulation stress status, male sex hormone could impact liver function, while female sex hormone could protect liver function (39). Thus, it is necessary to investigate the relationship of bilirubin with stroke in females and males.
The negative relationship between serum bilirubin and incidence of ischemic stroke may be explained by the anti-oxidative capability of the bilirubin (3), bilirubin induced inhibition of low-density lipoprotein (LDL) oxidation (40), anti-atherosclerotic capability of bilirubin and vascular structure and reactive pathways reported in recent years (41). Serum bilirubin has been confirmed to be a major contributor to total antioxidant capacity of the plasma (42). There is evidence showing that serum bilirubin can reduce the transport and generation of LDL, increase the transport of cholesterol from the blood vessels to the plasma, promote the lipolysis and bile clearance and serve as a physiological lipid antagonist affecting lipid metabolism (43). Animal studies have confirmed bilirubin may phosphorylated ERK1/2 via nNOS/NO/cGMP pathway, exerting neuroprotective effects.
Our study showed not only cross-sectional studies but also prospective studies indicated serum total bilirubin level was negatively related to the incidence of ischemic stroke, however they could not confirm the causal relationship: low serum bilirubin may be present before or after the ischemic stroke. MR uses genetic variants randomly allocated according to the Mendel’s second law without any preconception (44,45). MR refers to the use of genetic variants to develop causal inferences from observational data, if the variant genotype is associated with the phenotype and the variant genotype associated with the risk of interest through the phenotype. MR experiments are also warranted to investigate the potential causal implications of bilirubin in the stroke outcomes. Lee et al. suggested no causal relationship between a common genetic variant (rs6742078) of the UGT1A1 gene, robustly associated with increased circulating total bilirubin level, and risk for stroke in a Korean population, using the MR method (20). However, there are considerable ethnic differences in the genetic association of bilirubin levels (46). Besides, there is the potential existence of pleiotropy. Nevertheless, these results should be interpreted with caution, given the large sample size required for MR analysis and the plausibility of the instrumental variable assumption (47).
A variety of studies have investigated the relationship between serum bilirubin level and risk for cardiovascular diseases or conditions including atherosclerosis (48), hypertension (49), diabetes mellitus (50), metabolic syndrome (51), smoking (27) and excise (52). In patients with appropriate increase in the bilirubin level, bilirubin level may serve as an anti-thrombotic agent to inhibit platelet activation, reducing mortality (30). Heme-1 inducer, UGT1A1 gene antagonist, and drugs that can reduce liver glucuronidation activity and hepatocyte uptake may induce the mild to moderate increase in the bilirubin level, which may be employed as a promising strategy for the improvement of cardiovascular outcomes (53-56). This means that it is necessary to conduct more prospective studies with large sample size to confirm unresolved issues.
One of advantages in this study was the inclusion of both cross-sectional studies and prospective studies. In the prospective studies, the sample size was large (≥2,000) and the duration of follow-up was relatively long (max: 14 years) in 4 studies except for small sample size (<2,000) and short duration of follow-up (4.9 years) in two nested case-control studies. In addition, not only Asian population but also European population were studied in the studies included. Most included studies adjusted the potential risk factors, and the methodological assessment was satisfactory. In our study, publication bias was noted in studies on the relationship between serum bilirubin level and risk for stroke, but Trim and Fill method was used for adjustment. After adjustment, the results remained unchanged, suggesting that results of meta-analysis were reliable.
Of course, there were limitations in this study. First, the included studies could not confirm the causal relationship between bilirubin level and ischemic stroke/stroke. The subgroup analysis has its own limitations, and meta-regression analysis is infeasible to evaluate the relationship of bilirubin level with different variables of ischemic strokes. In addition, the number of studies on the relationship between bilirubin level and hemorrhagic stroke is still small, which limits the elucidation of relationship between bilirubin level and hemorrhagic stroke. Moreover, few studies focused on the direct bilirubin and/or indirect bilirubin, which limits the elucidation of relationship of stroke with bilirubin of different types.
Taken together, the cross-sectional studies and prospective studies included in this analysis support the negative relationship between serum bilirubin level and risk for ischemic stroke/stroke. The biological basis of this relationship is required to be confirmed in more studies with new methods (such as metabolomics) (53). In addition, more prospective studies with large sample size are needed to confirm the negative relationship between serum bilirubin level and risk for ischemic stroke.
Supplementary file 1
Search strategy
The following search strategy, using a combination of controlled vocabulary (MeSH) and free text terms, was used for MEDLINE (PubMed), and was modified for the other databases searched:
- “stroke”[MeSH Terms]
- “cerebral infarction”[MeSH Terms]
- “brain ischemia”[MeSH Terms]
- stroke* OR (brain infarct*) OR (cerebral infarct*) OR (brain ischemia) OR (brain ischaemia) OR (cerebral ischemia) OR (cerebral ischaemia) OR (cerebrovascular accident*) OR cva OR (cerebrovascular disorder*) OR (cerebrovascular disease*) OR (brain embol*) OR (brain thromb*) OR (cerebral embol*) OR (cerebral thromb*) OR (intracerebral embol*) OR (intracerebral thromb*) OR (intracranial embol*) OR (intracranial thromb*) OR apoplexy OR ictus
- “cerebrovascular disorders”[MeSH Terms]
- “cerebral hemorrhage”[MeSH Terms]
- “intracranial hemorrhages”[MeSH Terms]
- (brain haemorrhage*) OR (brain hemorrhage*) OR (cerebral haemorrhage*) OR (cerebral hemorrhage*) OR (intracerebral haemorrhage*) OR (intracerebral hemorrhage*) OR (intracranial haemorrhage*) OR (intracranial hemorrhage*)9. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 810. (bilirubin[mh] OR (bilirubin*[tiab]))11. 9 and 1012. limit 11 to human13. limit 11 to English
Acknowledgments
Funding: This study was supported by the Shanghai Science and Technology Committee Research Project (19401935700 and 17411967700) and Shanghai Advanced Chinese Medicine Industry Development Three-Year Action Plan [ZY(2018-2020)-FWTX-6013].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015;45:161-76. [Crossref] [PubMed]
- Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759-71. [Crossref] [PubMed]
- Mancuso C. Bilirubin and brain: A pharmacological approach. Neuropharmacology 2017;118:113-23. [Crossref] [PubMed]
- Kimm H, Yun JE, Jo J, et al. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke 2009;40:3422-7. [Crossref] [PubMed]
- Li RY, Cao ZG, Zhang JR, et al. Decreased serum bilirubin is associated with silent cerebral infarction. Arterioscler Thromb Vasc Biol 2014;34:946-51. [Crossref] [PubMed]
- Oda E, Kawai R. A possible cross-sectional association of serum total bilirubin with coronary heart disease and stroke in a Japanese health screening population. Heart Vessels 2012;27:29-36. [Crossref] [PubMed]
- Perlstein TS, Pande RL, Creager MA, et al. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999-2004. Am J Med 2008;121:781-788.e1. [Crossref] [PubMed]
- Kurzepa J, Bielewicz J, Stelmasiak Z, et al. Serum bilirubin and uric acid levels as the bad prognostic factors in the ischemic stroke. Int J Neurosci 2009;119:2243-9. [Crossref] [PubMed]
- Kunutsor SK, Bakker SJ, Gansevoort RT, et al. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol 2015;35:716-24. [Crossref] [PubMed]
- Li HY, Dai B, Shen GL, et al. Serum bilirubin levels in acute stroke in Chinese population: a meta-analysis. Int J Clin Exp Med 2017;10:905-12.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- van Dijk GM, Maneva M, Colpani V, et al. The association between vasomotor symptoms and metabolic health in peri- and postmenopausal women: a systematic review. Maturitas 2015;80:140-7. [Crossref] [PubMed]
- Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol 1996;144:610-21. [Crossref] [PubMed]
- Danesh J, Collins R, Appleby P, et al. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477-82. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Zhang TS, Zhong WZ. Performance of the Nonparametric Trim and Fill Method in Stata. J Evid Based Med 2009;9:240-2.
- Ekblom K, Marklund SL, Johansson L, et al. Bilirubin and UGT1A1*28 are not associated with lower risk for ischemic stroke in a prospective nested case-referent setting. Cerebrovasc Dis 2010;30:590-6. [Crossref] [PubMed]
- Lee SJ, Jee YH, Jung KJ, et al. Bilirubin and Stroke Risk Using a Mendelian Randomization Design. Stroke 2017;48:1154-60. [Crossref] [PubMed]
- Mahabadi AA, Lehmann N, Mohlenkamp S, et al. Association of bilirubin with coronary artery calcification and cardiovascular events in the general population without known liver disease: the Heinz Nixdorf Recall study. Clin Res Cardiol 2014;103:647-53. [Crossref] [PubMed]
- Jørgensen ME, Torp-Pedersen C, Finer N, et al. Association between serum bilirubin and cardiovascular disease in an overweight high risk population from the SCOUT trial. Nutr Metab Cardiovasc Dis 2014;24:656-62. [Crossref] [PubMed]
- Perlstein TS, Pande RL, Beckman JA, et al. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol 2008;28:166-72. [Crossref] [PubMed]
- Ren Y, Jin N, Hong T, et al. Interactive effect of serum uric acid and total bilirubin for cardiovascular disease in Chinese patients with type 2 diabetes. Sci Rep 2016;6:36437. [Crossref] [PubMed]
- Zhou X, Wang L, Liu H, et al. Serum antioxidant levels associated with subcortical ischemic vascular disease. Can J Neurol Sci 2014;41:375-81. [Crossref] [PubMed]
- Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 1994;40:18-23. [PubMed]
- Chan KH, O'Connell RL, Sullivan DR, et al. Plasma total bilirubin levels predict amputation events in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2013;56:724-36. [Crossref] [PubMed]
- Mashitani T, Hayashino Y, Okamura S, et al. Correlations between serum bilirubin levels and diabetic nephropathy progression among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT 5]). Diabetes Care 2014;37:252-8. [Crossref] [PubMed]
- Ong KL, Allison MA, Cheung BM, et al. The relationship between total bilirubin levels and total mortality in older adults: the United States National Health and Nutrition Examination Survey (NHANES) 1999-2004. PLoS One 2014;9:e94479. [Crossref] [PubMed]
- Kundur AR, Singh I, Bulmer AC. Bilirubin, platelet activation and heart disease: a missing link to cardiovascular protection in Gilbert's syndrome? Atherosclerosis 2015;239:73-84. [Crossref] [PubMed]
- Baranano DE, Rao M, Ferris CD, et al. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A 2002;99:16093-8. [Crossref] [PubMed]
- Maghzal GJ, Leck MC, Collinson E, et al. Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J Biol Chem 2009;284:29251-9. [Crossref] [PubMed]
- McDonagh AF. The biliverdin-bilirubin antioxidant cycle of cellular protection: Missing a wheel? Free Radic Biol Med 2010;49:814-20. [Crossref] [PubMed]
- Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043-6. [Crossref] [PubMed]
- Dohi K, Mochizuki Y, Satoh K, et al. Transient elevation of serum bilirubin (a heme oxygenase-1 metabolite) level in hemorrhagic stroke: bilirubin is a marker of oxidant stress. Acta Neurochir Suppl 2003;86:247-9. [PubMed]
- Huang FP, Xi G, Keep RF, et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg 2002;96:287-93. [Crossref] [PubMed]
- Lin SP, Lin PY, Jiang HL, et al. Is serum total bilirubin useful to differentiate cardioembolic stroke from other stroke subtypes? Neurol Res 2015;37:727-31. [Crossref] [PubMed]
- Sullivan JL. Iron and the genetics of cardiovascular disease. Circulation 1999;100:1260-3. [Crossref] [PubMed]
- Toth B, Yokoyama Y, Kuebler JF, et al. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch Surg 2003;138:1375-82. [Crossref] [PubMed]
- Lin JP, O'Donnell CJ, Schwaiger JP, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 2006;114:1476-81. [Crossref] [PubMed]
- McArdle PF, Whitcomb BW, Tanner K, et al. Association between bilirubin and cardiovascular disease risk factors: using Mendelian randomization to assess causal inference. BMC Cardiovasc Disord 2012;12:16. [Crossref] [PubMed]
- Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A 1988;85:9748-52. [Crossref] [PubMed]
- Bulmer AC, Verkade HJ, Wagner KH. Bilirubin and beyond: a review of lipid status in Gilbert's syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res 2013;52:193-205. [Crossref] [PubMed]
- Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1-22. [Crossref] [PubMed]
- Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 2004;33:30-42. [Crossref] [PubMed]
- Kang TW, Kim HJ, Ju H, et al. Genome-wide association of serum bilirubin levels in Korean population. Hum Mol Genet 2010;19:3672-8. [Crossref] [PubMed]
- Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol 2013;42:1134-44. [Crossref] [PubMed]
- Tatami Y, Suzuki S, Ishii H, et al. Impact of serum bilirubin levels on carotid atherosclerosis in patients with coronary artery disease. IJC Metabolic & Endocrine 2014;5:24-7. [Crossref]
- Chin HJ, Song YR, Kim HS, et al. The bilirubin level is negatively correlated with the incidence of hypertension in normotensive Korean population. J Korean Med Sci 2009;24 Suppl:S50-6. [Crossref] [PubMed]
- Wang J, Li Y, Han X, et al. Serum bilirubin levels and risk of type 2 diabetes: results from two independent cohorts in middle-aged and elderly Chinese. Sci Rep 2017;7:41338. [Crossref] [PubMed]
- Zhong P, Sun DM, Wu DH, et al. Serum total bilirubin levels are negatively correlated with metabolic syndrome in aged Chinese women: a community-based study. Braz J Med Biol Res 2017;50:e5252. [Crossref] [PubMed]
- Swift DL, Johannsen NM, Earnest CP, et al. Effect of different doses of aerobic exercise training on total bilirubin levels. Med Sci Sports Exerc 2012;44:569-74. [Crossref] [PubMed]
- Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222-31. [Crossref] [PubMed]
- McCarty MF. ''Iatrogenic Gilbert syndrome''--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses 2007;69:974-94. [Crossref] [PubMed]
- Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev 2009;17:99-111. [Crossref] [PubMed]
- Targher G. Risk of ischemic stroke and decreased serum bilirubin levels: is there a causal link? Arterioscler Thromb Vasc Biol 2014;34:702-4. [Crossref] [PubMed]