-
PDF
- Split View
-
Views
-
Cite
Cite
Hernán Cohen Arazi, David G. Doiny, Ricardo Spampinato Torcivia, Hugo Grancelli, Silvina V. Waldman, Carlos Nojek, María Cecilia Fornari, Juan Jose Badimon, Impaired anti-platelet effect of aspirin, inflammation and platelet turnover in cardiac surgery, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 6, June 2010, Pages 863–867, https://doi.org/10.1510/icvts.2009.229542
Close - Share Icon Share
Abstract
A reduced platelet inhibitory response to acetyl salicylic acid (ASA) has been associated with an increased risk of graft thrombotic occlusion after coronary artery bypass grafting (CABG). We performed a prospective, observational study of 18 patients on 100 mg/day ASA before and after CABG. We assessed antiplatelet response to ASA and its relationship with platelet turnover, inflammatory markers, and soluble thrombomodulin (sTM) levels. All patients showed optimal response to ASA preoperatively but had higher values during follow-up. Platelet aggregation and platelet count in the perioperative period were significantly associated (P=0.05). Platelet turnover was defined as the average daily turnover (ADTO). The lowest inhibitory value (28% of patients ≥6 Ω) was recorded at the same time of the highest platelet turnover (>10% daily in 77.77% of patients), one week after CABG. ADTO >10% was associated with an increased risk of platelet aggregation ≥6 Ω. Levels of sTM were significantly higher one week after CABG (median 13 vs. 3 ng/ml preoperatively, P=0.0011). There is a transient impairment in ASA antiplatelet effect after CABG related to an increased platelet turnover caused by the inflammatory process. This could be responsible for the high risk of occlusive thrombosis.
1. Introduction
Clinical success after coronary artery bypass grafting (CABG) depends upon graft patency [1, 2]. Acetyl salicylic acid (ASA) treatment has proved to be effective in preventing bypass occlusion after CABG [1–3].
Benefits of ASA treatment are greater during the first month after surgery, when platelet activation and thrombosis play a major role in graft occlusion [2]. However, some patients do not achieve an adequate anti-platelet effect despite ASA therapy. This ‘ASA-resistance’ could increase the risk for graft occlusion after CABG [2, 4–6].
Several mechanisms for inadequate response to ASA have been proposed, including increased platelet turnover rate. Inflammatory stimuli related to surgical trauma and cardiopulmonary bypass (CPB) have been associated with thrombocytosis [6]. Endothelial damage caused by the procedure itself could contribute to bypass graft occlusion in the early postoperative period [2, 7].
The present study assesses the platelet response to ASA therapy during the first month post-CABG and its relationship with platelet turnover and inflammatory markers. It also investigates the association between platelet reactivity and endothelial damage [assessed as levels of soluble thrombomodulin (sTM)] as possible causes for thrombotic graft occlusion after CABG.
2. Materials and methods
2.1. Subjects and laboratory tests
Eighteen consecutive patients undergoing elective CABG were included. Informed consent was obtained. The study was conducted in accordance with the Declaration of Helsinki and approved by the institution's Ethics Committee.
All patients were on chronic ASA (100 mg/day) until the day before CABG, and resumed the same treatment within 12 h after surgery.
Exclusion criteria were: CABG without CPB (n=5), consent withdrawal (n=2), concomitant carotid or valvular surgery, discontinuation or contraindication to ASA, treatment with clopidogrel or other non-steroidal intinflammatory drugs, acute myocardial infarction (MI) and creatinine clearance ≤30 ml/min.
We assessed levels of IL6, sTM, platelet count, white blood cell count (WBC) and platelet reactivity within the two days prior to surgery (t0), 24 h (t1), 72 h (t2), 7 days (t3), and one month post-CABG (t4).
T0 represents preoperative platelet aggregation; t1 shows possible effects of CPB and surgery on response to ASA. Samples for t3 were obtained one week after surgery, as different studies consider this moment particularly high-risk for bypass thrombosis, and in accordance with the hypothesis that IL6 stimulates platelet turnover through production of thrombopoietin. The latter peaks at day 3, and two to three days later platelet count reaches its maximum level [8]. Samples for t4 were collected one month after the CABG to obtain remote results.
Platelet reactivity was assessed by whole blood impedance (WBI) (Chronolog 590 D) using arachidonic acid (0.5 mM/l) as an agonist for platelet aggregation. Platelet aggregation was measured within 2 h of blood collection. Plasma levels of IL6 and sTM were assessed using specific ELISAs. Results are expressed as mean±S.D. Ω for platelet aggregation, pg/ml for IL6, ng/ml for sTM, n/mm3 for WBC and platelets. Platelet turnover was defined as the average daily turnover (ADTO), and was estimated as the percentage of change in the platelet count (as percentage of increase or decrease) divided by the number of days between the two determinations (e.g. patient no. 1: platelet count in t2 was 210,000/mm3 and in t3 it was 304,000/mm3, the variation between both is a 44.77% increase; number of days between t2 and t3=4 days; ADTO between t2 and t3=44.77/4=11.19%).
Blood samples were collected from 12 drug-free healthy subjects in each stage. The median aggregation values of these subjects were used as a reference value, representing no antiaggregation therapy.
2.2. Definitions
Systemic inflammatory response syndrome (SIRS): two or more of the following: temperature >38 °C or <36 °C, tachycardia, tachypnea with hypocapnia and WBC >12,000/mm3 or <4000/mm3, and requirement for vasopressors (norepinephrine >0.5 μg/kg/min) [9].
Significant bleeding: >500 ml at 1 h, >1000 ml in the first 3 h or >200 ml/h the first 5 h after the surgery.
Medical bleeding: alterations in the coagulation laboratory tests that could be solved by correcting these parameters.
Surgical bleeding: the patient needed surgery to solve the hemorrhage.
2.3. Statistics
χ2-test and Fisher's exact test were used for categorical variables. For continuous variables, we used either Student's t-test or Wilcoxon rank sum test depending on whether the data were normally distributed. For correlation, we used Spearman's coefficient.
3. Results
A total of 90 samples from 18 patients at times t0–t4 were collected and assessed.
Tables 1 and 2 show demographic and clinical characteristics of the patients and surgical details, respectively.
Demographics
| No. of patients | 18 |
| Age (years) median±S.D. | 64.9±9.6 |
| Sex: male (%) | 15 (83%) |
| Hypertension | 13 (72%) |
| Diabetes | 4 (22%) |
| Hypercholesterolemia | 14 (78%) |
| Current smoking | 6 (33%) |
| Previous smoking | 8 (45%) |
| BMI >30 | 1 (5%) |
| MI | 3 (17%) |
| PCI | 1 (5%) |
| CABG | 1(5%) |
| COPD | 2 (11%) |
| Treatment: | |
| • ACE inhibitors | • 11 (61%) |
| • Calcium antagonists | • 2 (11%) |
| • Statin | • 11 (61%) |
| LVEF | Median=63% |
| • Severely impaired=0 | |
| • Mildly impaired=4 (22%) |
| No. of patients | 18 |
| Age (years) median±S.D. | 64.9±9.6 |
| Sex: male (%) | 15 (83%) |
| Hypertension | 13 (72%) |
| Diabetes | 4 (22%) |
| Hypercholesterolemia | 14 (78%) |
| Current smoking | 6 (33%) |
| Previous smoking | 8 (45%) |
| BMI >30 | 1 (5%) |
| MI | 3 (17%) |
| PCI | 1 (5%) |
| CABG | 1(5%) |
| COPD | 2 (11%) |
| Treatment: | |
| • ACE inhibitors | • 11 (61%) |
| • Calcium antagonists | • 2 (11%) |
| • Statin | • 11 (61%) |
| LVEF | Median=63% |
| • Severely impaired=0 | |
| • Mildly impaired=4 (22%) |
MI, myocardial infarction; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; S.D., standard deviation.
Demographics
| No. of patients | 18 |
| Age (years) median±S.D. | 64.9±9.6 |
| Sex: male (%) | 15 (83%) |
| Hypertension | 13 (72%) |
| Diabetes | 4 (22%) |
| Hypercholesterolemia | 14 (78%) |
| Current smoking | 6 (33%) |
| Previous smoking | 8 (45%) |
| BMI >30 | 1 (5%) |
| MI | 3 (17%) |
| PCI | 1 (5%) |
| CABG | 1(5%) |
| COPD | 2 (11%) |
| Treatment: | |
| • ACE inhibitors | • 11 (61%) |
| • Calcium antagonists | • 2 (11%) |
| • Statin | • 11 (61%) |
| LVEF | Median=63% |
| • Severely impaired=0 | |
| • Mildly impaired=4 (22%) |
| No. of patients | 18 |
| Age (years) median±S.D. | 64.9±9.6 |
| Sex: male (%) | 15 (83%) |
| Hypertension | 13 (72%) |
| Diabetes | 4 (22%) |
| Hypercholesterolemia | 14 (78%) |
| Current smoking | 6 (33%) |
| Previous smoking | 8 (45%) |
| BMI >30 | 1 (5%) |
| MI | 3 (17%) |
| PCI | 1 (5%) |
| CABG | 1(5%) |
| COPD | 2 (11%) |
| Treatment: | |
| • ACE inhibitors | • 11 (61%) |
| • Calcium antagonists | • 2 (11%) |
| • Statin | • 11 (61%) |
| LVEF | Median=63% |
| • Severely impaired=0 | |
| • Mildly impaired=4 (22%) |
MI, myocardial infarction; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; S.D., standard deviation.
Surgical procedure characteristics
| No. of coronary anastomosis | Median – 3 [11p (61%)]=3 bp; |
| 3p (17%)=4 bp; 4p (22%)=2 bp | |
| Cross-clamp time (median) | 43 (39–51) min |
| CPB time | 76 min (25–75%=64–88) |
| Cardioplegic solution type | 100% Blood |
| Temperature | 5 (27.8%) mild hypothermia |
| No. of coronary anastomosis | Median – 3 [11p (61%)]=3 bp; |
| 3p (17%)=4 bp; 4p (22%)=2 bp | |
| Cross-clamp time (median) | 43 (39–51) min |
| CPB time | 76 min (25–75%=64–88) |
| Cardioplegic solution type | 100% Blood |
| Temperature | 5 (27.8%) mild hypothermia |
CPB, cardiopulmonary bypass.
Surgical procedure characteristics
| No. of coronary anastomosis | Median – 3 [11p (61%)]=3 bp; |
| 3p (17%)=4 bp; 4p (22%)=2 bp | |
| Cross-clamp time (median) | 43 (39–51) min |
| CPB time | 76 min (25–75%=64–88) |
| Cardioplegic solution type | 100% Blood |
| Temperature | 5 (27.8%) mild hypothermia |
| No. of coronary anastomosis | Median – 3 [11p (61%)]=3 bp; |
| 3p (17%)=4 bp; 4p (22%)=2 bp | |
| Cross-clamp time (median) | 43 (39–51) min |
| CPB time | 76 min (25–75%=64–88) |
| Cardioplegic solution type | 100% Blood |
| Temperature | 5 (27.8%) mild hypothermia |
CPB, cardiopulmonary bypass.
There were no deaths during the follow-up period; four patients experienced medical bleeding episodes; one patient experienced surgical bleeding.
All patients showed optimal response to ASA preoperatively when assessed by WBI (0 Ω). Interestingly, all patients showed some type of ‘variable response’ or ‘resistance’ during follow-up.
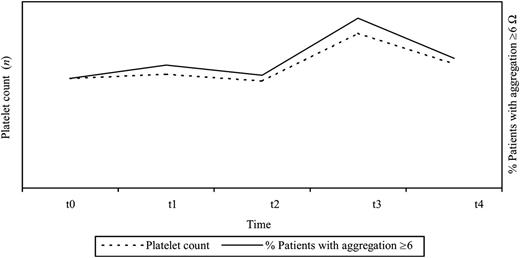
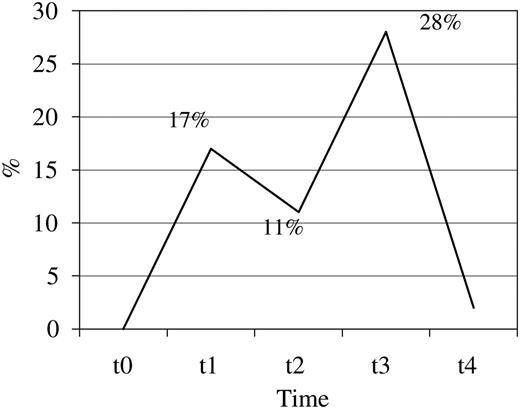
Out of 72 samples collected after surgery, 38 (53%) were ≥1 Ω. Median aggregation in healthy controls was 6 Ω (IC 25–75% 4–10 Ω). Twelve out of 72 samples of patients in the study (16.66%) had values above 6 Ω, mainly found at one week post-CABG, in which 28% of patients had this value (P=0.016 compared to t2) (Fig. 1) .
Percentage of patients with platelet aggregation ≥6 Ω by WBI, 28% of patients had values above 6 Ω one week after CABG. WBI, whole blood impedance; CABG, coronary artery bypass grafting.
Only two patients showed incomplete platelet inhibition one month after CABG. One of them had pericarditis in the postoperative period, still active one month later. The other patient presented an infected saphenectomy at t4. The infectious processes could be responsible for the observed thrombocytosis.
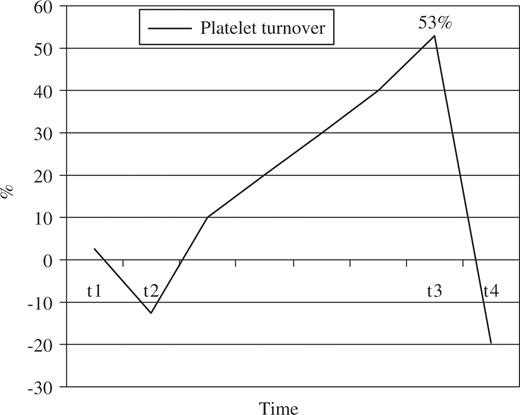
Platelet count showed a similar pattern to the one observed for platelet aggregation (Fig. 2 ), showing a slight increase (average of 1.5%) in t1, followed by a decrease to levels below the preoperative ones by t2 (–12.5% in average), then rising significantly 53% in t3, and finally returning to levels similar to those of the preoperative period at t4.
Platelet turnover (% of change) – average for the whole cohort of patients for each time. Platelet turnover picked in t3 (53% increase) and returned to levels similar to those of the preoperative period at t4.
Using ADTO as previously defined, there was a 1.5% increase in t1, a decrease of 6.25% between t2 and t3, and an average increase of 13.5% per day between t2 and t3.
The same two patients whose aggregation values remained inadequate by t4, also showed a significantly higher platelet count at the same time-points (over 55% higher than before CABG, in contrast with the rest of the patients who returned to levels similar to t0).
There was not a typical pattern in ASA response in the five patients who presented bleeding episodes.
IL6 peaked at t1, and then decreased to values similar to those recorded preoperatively at t4 (Fig. 3 ), except in the two patients with persistent inflammatory processes, in whom IL6 levels remained above the median (18 and 12 vs. 6 pg/ml, respectively).
At t3 the highest levels of sTM were detected, with values significantly higher [median 13 (5–38 ng/ml) vs. 3 ng/ml (1–7 ng/ml, P=0.0011)] than preoperatively, and different to healthy controls [13 (5–38 ng/ml) vs. 2.5 ng/ml (1–5 ng/ml, P=0.001)] (Fig. 4) .
Levels of sTM. Median of sTM [median (ng/ml), ±25–75%]. STM, soluble thrombomodulin.
We found a significant association between platelet aggregation levels and changes in platelet count in the perioperative period (P=0.05), recording the lowest inhibitory value (28% of patients ≥6 Ω) at the same time of the highest ADTO (>10% in 14 out of 18 patients, 77.77%) between t2 and t3 (Fig. 5 ). ADTO >10% was associated with an increased risk of impaired platelet inhibition (≥6 Ω).
Relationship between platelet count and percentage of patients with platelet aggregation ≥6 by WBI (two different scales). Association between platelet aggregation levels and changes in platelet count in the perioperative period (P=0.05). WBI, whole blood impedance.
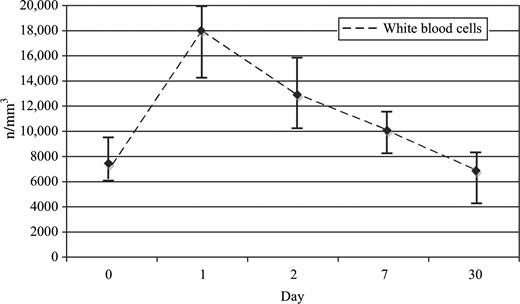
Levels of IL6 were highly associated with platelet count (P=0.013). There was a correlation between levels of IL6 and WBC count, with a Spearman correlation coefficient of 0.69 (P<0.0001) (Fig. 6) .
White blood cell count. Median±25–75% of white blood cells count (n/mm3).
According to the definition, in our study IL6 was a marker of SIRS when levels in t1 were above 100 pg/ml (P=0.034).
4. Discussion
This study shows important variations in platelet response to ASA after CABG. A dose of ASA 100 mg/day was enough to abolish AA-induced WBI-aggregation in all patients before CABG. Nevertheless, all patients registered transient increases in platelet reactivity despite continuing the same ASA treatment after surgery. These alterations were greater seven days after surgery, with 28% of patients showing values of platelet aggregation similar to those of healthy subjects not receiving ASA. One-month post-CABG, all patients except the two with signs of inflammation and thrombocytosis, recovered optimal platelet inhibition levels.
Reduced anti-platelet activity was significantly associated with high platelet turnover; ADTO >10% was significantly associated with a loss of the inhibitory effect of ASA. An association between increased IL6 levels and increased platelet count was also observed.
We observed a significant increase in sTM during the first week post-CABG, peaking at the same time as the lowest anti-platelet values are recorded. This phenomenon might account for the increased risk of occlusion of bypass grafts at this moment of the postoperative period.
Measurement of residual platelet reactivity by WBI in response to arachidonic acid is considered adequate to predict risk of cardiovascular events [6, 10].
The incidence of ASA resistance after CABG has been reported to range from 10 to >90% [2]. It is a transient phenomenon, more frequently seen during the first month after surgery, and some authors suggest that it may explain the elevated risk of graft thrombosis and death after CABG [2, 4, 5, 7].
The response of platelets to CPB is complex, with a reduction in platelet count from baseline during the first days, and then increasing to a higher count than preoperatively [4, 11, 12]. Enhanced platelet turnover which is intertwined with platelet hyperactivity, causes release of young platelets still able to form TX via uninhibited COX-1 and possibly through up-regulated COX-2 generating critical amounts of TX despite treatment [6, 10–13].
Studies in normal healthy subjects have indicated that platelet TX synthesis needs to be blocked to >90% to achieve efficient platelet inhibition, and a 5-day treatment with oral ASA (100 mg/day) is sufficient to significantly inhibit arachidonic acid-induced platelet aggregation [11, 12].
After CPB an enhanced platelet turnover may compromise the inhibitory effect of ASA by generating a number of platelets than cannot be effectively inhibited by the usual daily dose of 100 mg ASA [2, 4, 9, 13]. If immediately before ingestion of the daily ASA dose a significant amount (≥10%) of circulating platelets are competent for TX formation, circadian inhibition of platelet function may be incomplete, considering that plasma-half-life of aspirin is <30 min [4, 11].
CPB causes systemic inflammatory response and platelet activation [10]. Plasma levels of IL6 significantly correlate with systemic inflammatory response and reflect the severity of acute inflammation [14].
Inflammatory disorders are commonly associated with thrombocytosis; IL6 is able to stimulate megakaryocyte proliferation and increase platelet count [8]. Endothelial cells modulate coagulation to ensure microvascular flow through the expression of thrombomodulin (TM) that has anticoagulant effects. In severe inflammatory processes cytokines activate endothelial cells and cause a leak of TM. Elevated sTM levels have been observed in a number of conditions associated with endothelial stress, including trauma and SIRS, and is associated with high mortality [15].
5. Limitations
The major limitation of this study is the reduced number of patients enrolled. Because we consider that the surgical procedure itself impacts on platelet aggregation, we conducted our study in patients operated by the same surgical team. The reduced number of patients does not allow for assessing differences in the rates of events between patients. However, as five samples were obtained from each patient, conclusions can be drawn from the analysis of the laboratory results.
Samples were not collected on a daily basis, so we created an index to calculate an ADTO. We consider that an ADTO above 10% means that real daily turnover must have been >10% at least once in that period.
6. Conclusions
There is a transient impairment in ASA antiplatelet effect after CABG related to an increased platelet turnover caused by the inflammatory process secondary to the surgical procedure itself. This process could be, at least in part, responsible for the high-risk of occlusive thrombosis reported by previous studies. Inflammatory markers and platelet aggregation could be used as a guide to establish the dose of ASA needed for each individual patient. An alternative surrogate marker could be the monitoring of WBC and platelet count.
The homogeneity of the results, despite the reduced number of patients enrolled in the study, strongly support the notion that 100 mg ASA/day may not be enough to achieve optimal platelet inhibition in the first days after CABG. The feasibility of the administration of 300 mg ASA/day vs. the administration of 100 mg given three times a day is an ongoing investigation by our group.
Presented at European Congress of Cardiology, Barcelona, October 2009.
Founding: Research Department FLENI.
Claudio Pensa, Adrián Lescano, Marcelo Casey, R. Martingano, R. García Elezequi, Walter Rodriguez, María Fernanda Cobas; Vanina Berardi; María Clara Horsburgh.



![Levels of IL6. Median of IL6 [median (pg/ml), ±25–75%].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icvts/10/6/10.1510_icvts.2009.229542/2/m_863fig3.jpeg?Expires=1716206445&Signature=IhEXDoZKm0rjLCdte39Z6xbTdqBXlzjbklj53zsQfn2vq0iQHZurbwznBw1mHDyyBlFnx8P4-3O6vLXUhq~lfAzAqQgHKenH-Ll~K~jh2alT3-KgA2SYsslU1oWP6yqqR3KOesPPYbS-kRQW~XdAziwblMw69iq1Uq9spd2uB6WNnUnB24RTn-Miu~MU69yKaX3UagyHTmMukKCRZZZVmNYUuVti6DH8~HdvVZ~BLhcl09xbTXsQU0iFcro-iExnJYPYvKpiBOV4XARbl1P0v41FnWIeAvp5fHvCDmWReDnbVWZzn4eQgiabdXKrmUf~0q5U-pADnnp5-hScSDMd8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Levels of sTM. Median of sTM [median (ng/ml), ±25–75%]. STM, soluble thrombomodulin.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icvts/10/6/10.1510_icvts.2009.229542/2/m_863fig4.jpeg?Expires=1716206445&Signature=euUHtF9S9VqIPcsUpIk6HHYYlXsczPVIHJ7TwVAjDtz3iKH4D-CqcZhs7A8V2obDOwe-G0z1BX9iQ5C2L92JUDlmOErWXvI1uRWnAc-yITq3lNJBRC019EO40psc8aQcBmMQLk2gpwZ2zZlWDkoxA1l~D2-ORtvx2sE~AfrpERY8ZpzDPqZxWIRG8cJUIdkAlqxrwQyu2-h4fxQDXHuKewsC4m572A3FrSuIP6~AjdEnw6FOKwZEgq2xxRti~K1E4xv4Qe2e03IAEL5k3Rr7sKidebF-pqm6GdYGmTpONBFiRQASafVn0eoFpl36bY-2ymeayuWmbRxC7raMS2GVSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)