Abstract
Background
The diagnostic accuracy of biliary cytology is limited. A novel sandwich enzyme-linked immunosorbent assay that combined Wisteria floribunda agglutinin (WFA) and anti-sialylated mucin 1 (MUC1) monoclonal antibody to target bile samples was recently developed. This study was designed to verify the diagnostic accuracy of WFA-sialylated MUC1 as a sensitive biliary biomarker for human biliary tract cancer.
Methods
Bile samples from 27 patients with benign disease and 174 patients with biliary tract cancer were analyzed. A receiver-operated characteristic curve analysis for biliary WFA-sialylated MUC1 and serum CA19-9 levels was performed to determine the cutoff value for the prediction of the presence of biliary tract cancer.
Results
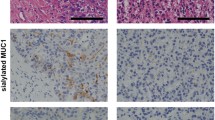
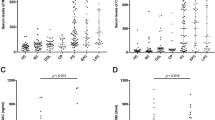
Biliary WFA-sialylated MUC1 levels were significantly higher in the biliary tract cancer group compared with the benign group (P < 0.001). The cutoff value of WFA-sialylated MUC1 for discriminating biliary tract cancer was 10.5. The sensitivity of WFA-sialylated MUC1 in discriminating biliary tract cancer was much higher (82.2 %) than that of cytology (23.6 %) when this cutoff value was used. The cutoff value of serum CA19-9 for discriminating biliary tract cancer was 38 IU/L in the same cohort. All patients with biliary WFA-sialylated MUC1 and serum CA19-9 above the cutoff values had biliary tract cancer, and no patient with benign disease was categorized in this group.
Conclusions
Biliary WFA-sialylated MUC1 is a useful biomarker for the differentiation of biliary tract cancer. The sensitivity of WFA-sialylated MUC1 was clearly higher than that of biliary cytology. Further data collection is necessary to validate the clinical usefulness of this biomarker.



Similar content being viewed by others
References
Tsuchiya T, Yokoyama Y, Ebata T, Igami T, Sugawara G, Kato K, et al. Randomized controlled trial on timing and number of sampling for bile aspiration cytology. J Hepatobiliary Pancreat Sci. 2014;21(6):433–8.
Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26(5):474–9.
Narimatsu H, Iwasaki H, Nakayama F, Ikehara Y, Kudo T, Nishihara S, et al. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58(3):512-8.
Matsuda A, Kuno A, Kawamoto T, Matsuzaki H, Irimura T, Ikehara Y, et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology. 2010;52(1):174–82.
Takeuchi H, Kato K, Denda-Nagai K, Hanisch FG, Clausen H, Irimura T. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialyl alpha 2-3galactosyl beta 1-3 N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J Immunol Methods. 2002;270(2):199–209.
Hattori M, Nagino M, Ebata T, Kato K, Okada K, Shimoyama Y. Prospective study of biliary cytology in suspected perihilar cholangiocarcinoma. Br J Surg. 2011;98(5):704–9.
Nakayama A, Imamura H, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S. Proximal bile duct stricture disguised as malignant neoplasm. Surgery. 1999;125(5):514–21.
Corvera CU, Blumgart LH, Darvishian F, Klimstra DS, DeMatteo R, Fong Y, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201(6):862–9.
Wakai T, Shirai Y, Sakata J, Maruyama T, Ohashi T, Korira PV, et al. Clinicopathological features of benign biliary strictures masquerading as biliary malignancy. Am Surg. 2012;78(12):1388–91.
Gerhards MF, Vos P, van Gulik TM, Rauws EA, Bosma A, Gouma DJ. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg. 2001;88(1):48–51.
Are C, Gonen M, D’Angelica M, DeMatteo RP, Fong Y, Blumgart LH, et al. Differential diagnosis of proximal biliary obstruction. Surgery. 2006;140(5):756–63.
Fujita T, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, et al. Incidence, clinical presentation and pathological features of benign sclerosing cholangitis of unknown origin masquerading as biliary carcinoma. J Hepatobil Pancreat Sci. 2010;17(2):139–46.
Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Nagino M. Review of hepatopancreatoduodenectomy for biliary cancer: an extended radical approach of Japanese origin. J Hepatobiliary Pancreat Sci. 2014;21(8):550–5.
Huang L, Chen W, Liang P, Hu W, Zhang K, Shen S, et al. Serum CYFRA 21-1 in biliary tract cancers: a reliable biomarker for gallbladder carcinoma and intrahepatic cholangiocarcinoma. Dig Dis Sci. 2015;60(5):1273–83.
Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10(3):427–32.
Akdogan M, Parlak E, Kayhan B, Balk M, Saydam G, Sahin B. Are serum and biliary carcinoembryonic antigen and carbohydrate antigen19-9 determinations reliable for differentiation between benign and malignant biliary disease? Turk J Gastroenterol. 2003;14(3):181-4.
Ince AT, Yildiz K, Baysal B, Danalıoğlu A, Kocaman O, Tozlu M, et al. Roles of serum and biliary CEA, CA19-9, VEGFR3, and TAC in differentiating between malignant and benign biliary obstructions. Turk J Gastroenterol. 2014;25(2):162–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Yokoyama, Y., Ebata, T. et al. Verification of WFA-Sialylated MUC1 as a Sensitive Biliary Biomarker for Human Biliary Tract Cancer. Ann Surg Oncol 23, 671–677 (2016). https://doi.org/10.1245/s10434-015-4878-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4878-4