-
PDF
- Split View
-
Views
-
Cite
Cite
Kristin A. Bradley, Ian F. Pollack, Joel M. Reid, Peter C. Adamson, Matthew M. Ames, Gilbert Vezina, Susan Blaney, Percy Ivy, Tianni Zhou, Mark Krailo, Gregory Reaman, Minesh P. Mehta, Motexafin gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: A Children's Oncology Group phase I study, Neuro-Oncology, Volume 10, Issue 5, October 2008, Pages 752–758, https://doi.org/10.1215/15228517-2008-043
Close - Share Icon Share
Abstract
The purpose of this study was to determine the dose-limiting toxicities, maximum tolerated dose, pharmacokinetics, and intratumor and brain distribution of motexafin gadolinium (MGd) with involved field radiation therapy in children with newly diagnosed intrinsic pontine gliomas. MGd was administered as a 5-min intravenous bolus 2–5 h prior to standard radiation. The starting dose was 1.7 mg/kg. After first establishing that 5 doses/week for 6 weeks was tolerable, the dose of MGd was escalated until dose-limiting toxicity was reached. Radiation therapy was administered to 54 Gy in 30 once-daily fractions. Forty-four children received MGd at doses of 1.7 to 9.2 mg/kg daily prior to radiation therapy for 6 weeks. The maximum tolerated dose was 4.4 mg/kg. The primary dose-limiting toxicities were grade 3 and 4 hypertension and elevations in serum transaminases. Median elimination half-life and clearance values were 6.6 h and 25.4 ml/kg/h, respectively. The estimated median survival was 313 days (95% confidence interval, 248–389 days). The maximum tolerated dose of MGd and the recommended phase II dose was 4.4 mg/kg when administered as a daily intravenous bolus in conjunction with 6 weeks of involved field radiation therapy for pediatric intrinsic pontine gliomas.
The outcome for children diagnosed with brainstem gliomas, which comprise 10%–20% of all pediatric CNS tumors, is poor.1–3 Diffuse intrinsic pontine tumors account for approximately 70%–80% of all brainstem gliomas and confer a poorer prognosis than the exophytic and focal types.1,4–6 The primary treatment approach for intrinsic pontine gliomas, radiation therapy using once-daily fractions to 54 to 60 Gy, results in a median survival of 8–12 months, with 2-year overall survival rates of 10% or less.6–12
Local treatment failure within the radiation field remains the predominant reason for treatment failure. Many investigational approaches to treatment have been studied, including twice-daily hyperfractionated radiation therapy affording dose escalation to 72–78 Gy,9,10,13–15 without improvements in outcome. It is uncertain whether this innate radioresistance of intrinsic pontine gliomas is due to enhanced repair mechanisms or failure of radiation-induced cell death.
Recent investigational efforts have focused on the addition of radiosensitizing agents to local radiation to improve tumor control.16–20 Motexafin gadolinium (MGd) is an expanded metalloporphyrin that localizes in tumors with minimal normal tissue incorporation. In preclinical models, MGd has a radiation sensitizer enhancement ratio of approximately 2, primarily through depletion of repair enzymes including thioredoxin reductase.21 The presence of gadolinium also permits an analysis of drug distribution using MRI.
We report the results of a phase I study conducted by the Children's Oncology Group (COG) investigating the use of MGd in conjunction with involved field radiation therapy for children with newly diagnosed intrinsic pontine gliomas.
Patients and Methods
Patients
Patients younger than 21 years of age with diffuse intrinsic brainstem gliomas were eligible for this trial. Histologic verification was not required. Patients with tumors that intrinsically involved the pons (greater than 50% intra-axial), the pons and medulla, the pons and midbrain, or the entire brainstem or that contiguously involved the thalamus or upper cervical cord were eligible. Other eligibility criteria included a Karnofsky or Lansky performance score of ⩾50; adequate bone marrow function (absolute neutrophil count ⩾1,000 μl, platelet count ⩾100,000 μl [transfusion independent], and hemoglobin ⩾10 g/dl); adequate renal function (serum creatinine ⩽1.5 times normal or a radioisotope glomerular filtration rate ⩾70 ml/min/1.73 m2); adequate liver function (bilirubin ⩽1.5 times the upper limit of normal and alanine aminotransferase ⩽1.5 times normal); and a life expectancy of greater than 8 weeks. Patients who had received prior cranial radiotherapy, MGd, or systemic chemotherapy, who were known to have G6PD deficiency, or who were pregnant or breast-feeding were ineligible. Women and men of childbearing potential had to agree to use an effective method of contraception while receiving protocol therapy.
Institutional review boards at the participating institutions approved the study. Informed consent was obtained from patients ⩾18 years, and permission was obtained from parents or legal guardians of children, with child assent when appropriate, according to individual institutional policies.
Drug Administration
MGd was supplied by Pharmacyclics, Inc. (Sunnyvale, CA, USA), and distributed by the National Cancer Institute, Division of Cancer Treatment and Diagnosis, as a dark green, 2.5 mg/ml solution in single-use 50-ml vials. The solution was isotonic in 5% mannitol, adjusted to pH 5.4 with a small amount of acetic acid. MGd was administered by i.v. push over 5 min. Radiation therapy was administered within 2 to 5 h after completion of the infusion. Since MGd was not formulated with preservatives, it was administered within 8 h once drawn from the vials. Patients were to be well hydrated orally before the administration of MGd. If the patient was noted to be tachycardic or had other signs of dehydration, i.v. hydration with 250–1,000 ml of an appropriate i.v. fluid was recommended before treatment.
Study Design
MGd dose escalation was accomplished initially by increasing the duration and frequency of administration and then by increasing the dose of drug. In the initial cohort, MGd was administered daily for 5 consecutive days each week for 3 weeks. The second cohort received MGd 3 days per week (Monday, Wednesday, and Friday) for 6 weeks. All subsequent cohorts received MGd daily for 5 consecutive days (Monday–Friday) each week for 6 weeks. Concurrent use of corticosteroids was permitted.
Radiation therapy was administered at a daily dose of 1.8 Gy for 30 fractions over 6 weeks, for a total dose of 54 Gy. The treatment fields encompassed the entire tumor plus margin. Three-dimensional planning was encouraged but not mandated, and MR-based contouring was employed for treatment planning. Normal critical structure tolerances were respected, including the optic chiasm and spinal cord.
At least three patients were studied at each dose level. When none of these three patients experienced dose-limiting toxicity (DLT), the dose was escalated to the next level. When one patient experienced DLT, the cohort was expanded up to six patients at that dose level. When one or more of these three to six additional patients experienced DLT, the maximum tolerated dose (MTD) was exceeded; up to three more patients were then treated at the next lower dose level. The MTD was the dose level at which none of six or one of six patients experienced DLT with at least two of three or two of six patients encountering DLT at the next higher dose.
Assessment for toxicity using National Cancer Institute (NCI) Common Toxicity Criteria (version 2.0) was performed weekly during treatment, at weeks 7, 8, 12, and 24 postinitiation of therapy, and then every 3 months thereafter up to 36 months. Hematologic DLT was defined as any grade 4 hematologic toxicity that persisted for more than 7 days or that required platelet transfusions for a period of time exceeding 7 days during the assigned weeks of concurrent chemotherapy and radiation therapy. Nonhematologic DLT was defined as any grade 3 or 4 nonhematologic toxicity with the exception of grade 3 nausea and/or vomiting that was controlled within 7 days. Survival was analyzed as a secondary end point.
Pharmacokinetic Analysis
Blood samples were collected prior to drug administration and 0.25, 0.5, 1, 2, 4, 8, 24, 48, and 96 h after the first dose. The 24-, 48-, and 96-h specimens were drawn before the MGd dose scheduled for that day. The blood samples (3–5 ml) were drawn into trace metal serum tubes and allowed to stand at room temperature for 45–60 min. After separation by centrifugation (1,000 g for 10 min), serum was decanted into a metal-free tube and stored at -70°C. Urine was collected for 24 h after the first MGd dose.
MGd serum concentrations were expressed in elemental Gd molar equivalents. Elemental Gd was assayed by inductively coupled plasma mass spectrometry. After 1:25 dilution with 1% nitric acid containing 100 ng/ml terbium, the internal standard, standards, quality control, and unknown samples were infused into a Perkin-Elmer Sciex Elan DRC II (Wellesley, MA, USA) using a Gilson AS90 autosampler operating at a rate of 0.5 ml/min. Gd was expressed as the sum of Gd species detected at 155, 156, 157, 158, and 160 atomic mass units (amu) using a program that sweeps 1–263 amu 50 times/reading. Gd standards (1–10,000 ng/ml in 1% nitric acid) were utilized to confirm the linearity of the assay (r ⩾ 0.999). Each unknown was determined in duplicate.
MGd plasma concentration-time data were fit by noncompartmental analysis using WinNonlin Pro version 4.1 (Pharsight, Mountain View, CA, USA). For noncompartmental analysis, the terminal elimination rate constant was determined by least-squares regression of the ln (serum concentration) of time data using the 4–24 h or 4–48 h time points.
MR Analysis
Both Gd-enhanced and unenhanced MRI scans were performed at baseline and day 30. A scoring system was used to categorize uptake of MGd on these MR images: (1) None (for the day 30 MRI, mild diffuse increase in T1 intensity compared to baseline with a decrease in T2 signal intensity, consistent with change in hydration rather than MGd uptake, was categorized as “none”); (2) Minimal: hypointense to isointense to normal white matter; (3) Moderate: isointense to mildly hyperintense to normal white matter; (4) Intense: moderately to markedly hyperintense to normal white matter; or (5) Could not be evaluated/not available: either MRI study not performed, not available for review, or patient removed from protocol therapy prior to day 30. In addition, the amount of uptake within the tumor was assessed relative to the tumor volume and categorized as <50% tumor volume or >50% tumor volume. Tumor volume was a volumetric measurement based on axial T2/FLAIR and T1 sequences.
MRI with and without Gd enhancement was also performed at 3, 6, and 12 months following treatment initiation and at the discretion of the treating physician thereafter.
Results
Forty-four patients, median age 6 years (range 2–20 years), were enrolled in the study (ADVL 09712) between August 1999 and March 2005 (Table 1). No patient was declared ineligible. Three patients were not fully evaluable for toxicity, one each at the 1.7, 5.5, and 7.1 mg/kg/day dose levels. One patient developed early progressive disease and required nonprotocol therapy, one patient missed 17% of therapy days with the actual dose received closer to the preceding dose level and one patient developed nonneutropenic pneumocystis pneumonia and came off study. The estimated median survival of patients was 313 days (95% confidence interval, 248–389 days).
Patient characteristics (n = 44)
| Characteristic . | Number (%) . |
|---|---|
| Age at enrollment, years | |
| &nbps;&nbps;&nbps;&nbps;Median | 6 |
| &nbps;&nbps;&nbps;&nbps;Range | 2–20 |
| Gender | |
| &nbps;&nbps;&nbps;&nbps;Male | 16 (36.4) |
| &nbps;&nbps;&nbps;&nbps;Female | 28 (63.6) |
| Race | |
| &nbps;&nbps;&nbps;&nbps;White | 34 (77.2) |
| &nbps;&nbps;&nbps;&nbps;Black | 3 (6.7) |
| &nbps;&nbps;&nbps;&nbps;Other/unknown | 7 (16.1) |
| Karnofsky Score | |
| &nbps;&nbps;&nbps;&nbps;100% | 6 (13.7) |
| &nbps;&nbps;&nbps;&nbps;80%–90% | 21 (47.7) |
| &nbps;&nbps;&nbps;&nbps;60%–70% | 17 (38.9) |
| Characteristic . | Number (%) . |
|---|---|
| Age at enrollment, years | |
| &nbps;&nbps;&nbps;&nbps;Median | 6 |
| &nbps;&nbps;&nbps;&nbps;Range | 2–20 |
| Gender | |
| &nbps;&nbps;&nbps;&nbps;Male | 16 (36.4) |
| &nbps;&nbps;&nbps;&nbps;Female | 28 (63.6) |
| Race | |
| &nbps;&nbps;&nbps;&nbps;White | 34 (77.2) |
| &nbps;&nbps;&nbps;&nbps;Black | 3 (6.7) |
| &nbps;&nbps;&nbps;&nbps;Other/unknown | 7 (16.1) |
| Karnofsky Score | |
| &nbps;&nbps;&nbps;&nbps;100% | 6 (13.7) |
| &nbps;&nbps;&nbps;&nbps;80%–90% | 21 (47.7) |
| &nbps;&nbps;&nbps;&nbps;60%–70% | 17 (38.9) |
Patient characteristics (n = 44)
| Characteristic . | Number (%) . |
|---|---|
| Age at enrollment, years | |
| &nbps;&nbps;&nbps;&nbps;Median | 6 |
| &nbps;&nbps;&nbps;&nbps;Range | 2–20 |
| Gender | |
| &nbps;&nbps;&nbps;&nbps;Male | 16 (36.4) |
| &nbps;&nbps;&nbps;&nbps;Female | 28 (63.6) |
| Race | |
| &nbps;&nbps;&nbps;&nbps;White | 34 (77.2) |
| &nbps;&nbps;&nbps;&nbps;Black | 3 (6.7) |
| &nbps;&nbps;&nbps;&nbps;Other/unknown | 7 (16.1) |
| Karnofsky Score | |
| &nbps;&nbps;&nbps;&nbps;100% | 6 (13.7) |
| &nbps;&nbps;&nbps;&nbps;80%–90% | 21 (47.7) |
| &nbps;&nbps;&nbps;&nbps;60%–70% | 17 (38.9) |
| Characteristic . | Number (%) . |
|---|---|
| Age at enrollment, years | |
| &nbps;&nbps;&nbps;&nbps;Median | 6 |
| &nbps;&nbps;&nbps;&nbps;Range | 2–20 |
| Gender | |
| &nbps;&nbps;&nbps;&nbps;Male | 16 (36.4) |
| &nbps;&nbps;&nbps;&nbps;Female | 28 (63.6) |
| Race | |
| &nbps;&nbps;&nbps;&nbps;White | 34 (77.2) |
| &nbps;&nbps;&nbps;&nbps;Black | 3 (6.7) |
| &nbps;&nbps;&nbps;&nbps;Other/unknown | 7 (16.1) |
| Karnofsky Score | |
| &nbps;&nbps;&nbps;&nbps;100% | 6 (13.7) |
| &nbps;&nbps;&nbps;&nbps;80%–90% | 21 (47.7) |
| &nbps;&nbps;&nbps;&nbps;60%–70% | 17 (38.9) |
Toxicity
Nine dose levels were studied (Table 2). Of note, at the time patients were being enrolled to the fourth dose level, adult studies of MGd demonstrated the safety of approximately twice the dose then under evaluation in the pediatric study. Therefore, following a protocol amendment, the fifth dose level represented an approximate 80% increase over the fourth dose level.
Dose-limiting toxicities observed by assigned dose level
| Dose Level (mg/kg/day)* . | Total Dose over 6 Weeks (mg/kg) . | Number Entered . | Number Evaluable . | Number with DLT . | Description of DLT . |
|---|---|---|---|---|---|
| 1.7 M–F, 3 weeks | 25.5 | 4 | 4 | 1 | Catheter-related infection |
| 1.7 MWF, 6 weeks | 30.6 | 4 | 4 | 0 | |
| 1.7 M–F, 6 weeks | 51 | 5 | 4 | 0 | |
| 1.9 M–F, 6 weeks* | 57 | 6 | 6 | 1 | Fibrinogen |
| 3.4 M–F, 6 weeks | 102 | 4 | 4 | 0 | |
| 4.4 M–F, 6 weeks | 132 | 6 | 6** | 1 | Elevated hepatic transaminases, hepatic enlargement |
| 5.5 M–F, 6 weeks | 165 | 7 | 6** | 2 | Elevated hepatic transaminases, hypertension |
| 7.1 M–F, 6 weeks | 213 | 6 | 5** | 2 | Hypertension, headache |
| 9.2 M–F, 6 weeks | 276 | 2 | 2 | 2 | Hypertension, urticaria, red cell transfusion |
| Dose Level (mg/kg/day)* . | Total Dose over 6 Weeks (mg/kg) . | Number Entered . | Number Evaluable . | Number with DLT . | Description of DLT . |
|---|---|---|---|---|---|
| 1.7 M–F, 3 weeks | 25.5 | 4 | 4 | 1 | Catheter-related infection |
| 1.7 MWF, 6 weeks | 30.6 | 4 | 4 | 0 | |
| 1.7 M–F, 6 weeks | 51 | 5 | 4 | 0 | |
| 1.9 M–F, 6 weeks* | 57 | 6 | 6 | 1 | Fibrinogen |
| 3.4 M–F, 6 weeks | 102 | 4 | 4 | 0 | |
| 4.4 M–F, 6 weeks | 132 | 6 | 6** | 1 | Elevated hepatic transaminases, hepatic enlargement |
| 5.5 M–F, 6 weeks | 165 | 7 | 6** | 2 | Elevated hepatic transaminases, hypertension |
| 7.1 M–F, 6 weeks | 213 | 6 | 5** | 2 | Hypertension, headache |
| 9.2 M–F, 6 weeks | 276 | 2 | 2 | 2 | Hypertension, urticaria, red cell transfusion |
Abbreviations: DLT, dose-limiting toxicity; M, Monday; F, Friday; W, Wednesday
Protocol amended to increase in a greater than 30% increment from 1.9 to 3.4 mg/kg/day based on adult tolerability data
Initial group of three patients at these dose levels did not experience DLT; DLTs were observed during subsequent de-escalations and cohort expansion
Dose-limiting toxicities observed by assigned dose level
| Dose Level (mg/kg/day)* . | Total Dose over 6 Weeks (mg/kg) . | Number Entered . | Number Evaluable . | Number with DLT . | Description of DLT . |
|---|---|---|---|---|---|
| 1.7 M–F, 3 weeks | 25.5 | 4 | 4 | 1 | Catheter-related infection |
| 1.7 MWF, 6 weeks | 30.6 | 4 | 4 | 0 | |
| 1.7 M–F, 6 weeks | 51 | 5 | 4 | 0 | |
| 1.9 M–F, 6 weeks* | 57 | 6 | 6 | 1 | Fibrinogen |
| 3.4 M–F, 6 weeks | 102 | 4 | 4 | 0 | |
| 4.4 M–F, 6 weeks | 132 | 6 | 6** | 1 | Elevated hepatic transaminases, hepatic enlargement |
| 5.5 M–F, 6 weeks | 165 | 7 | 6** | 2 | Elevated hepatic transaminases, hypertension |
| 7.1 M–F, 6 weeks | 213 | 6 | 5** | 2 | Hypertension, headache |
| 9.2 M–F, 6 weeks | 276 | 2 | 2 | 2 | Hypertension, urticaria, red cell transfusion |
| Dose Level (mg/kg/day)* . | Total Dose over 6 Weeks (mg/kg) . | Number Entered . | Number Evaluable . | Number with DLT . | Description of DLT . |
|---|---|---|---|---|---|
| 1.7 M–F, 3 weeks | 25.5 | 4 | 4 | 1 | Catheter-related infection |
| 1.7 MWF, 6 weeks | 30.6 | 4 | 4 | 0 | |
| 1.7 M–F, 6 weeks | 51 | 5 | 4 | 0 | |
| 1.9 M–F, 6 weeks* | 57 | 6 | 6 | 1 | Fibrinogen |
| 3.4 M–F, 6 weeks | 102 | 4 | 4 | 0 | |
| 4.4 M–F, 6 weeks | 132 | 6 | 6** | 1 | Elevated hepatic transaminases, hepatic enlargement |
| 5.5 M–F, 6 weeks | 165 | 7 | 6** | 2 | Elevated hepatic transaminases, hypertension |
| 7.1 M–F, 6 weeks | 213 | 6 | 5** | 2 | Hypertension, headache |
| 9.2 M–F, 6 weeks | 276 | 2 | 2 | 2 | Hypertension, urticaria, red cell transfusion |
Abbreviations: DLT, dose-limiting toxicity; M, Monday; F, Friday; W, Wednesday
Protocol amended to increase in a greater than 30% increment from 1.9 to 3.4 mg/kg/day based on adult tolerability data
Initial group of three patients at these dose levels did not experience DLT; DLTs were observed during subsequent de-escalations and cohort expansion
The two most common DLTs observed were hypertension (four episodes) and elevations in serum transaminases (four episodes). A total of 14 patients experienced DLT (Table 2). At the 4.4, 5.5, and 7.1 mg/kg/day dose levels, none of the initial three patients entered experienced a DLT. However, at the 9.2 mg/kg/day dose level, two of two patients experienced DLT, resulting in sequential dose de-escalations and cohort expansions at the 7.1, 5.5, and ultimately 4.4 mg/kg/day dose levels (Table 2). At the 4.4 mg/kg/day dose level, only one of six patients experienced DLT, establishing the MTD and recommended phase II dose.
No patients experienced grade 3 or 4 neutropenia or thrombocytopenia attributable to MGd while receiving protocol therapy; two patients required transfusion of packed red blood cells while on protocol, one patient each at 1.9 mg/kg/day and 9.2 mg/kg/day dose levels. Non-DLTs observed in more than 10% of patients are listed in Table 3.
Number of patients (%) with non–dose-limiting non-hematologic toxicities related to protocol therapy and observed in more than 10% of 41 evaluable patients
| . | Maximum Grade across the Course . | . | |
|---|---|---|---|
| Toxicity Type . | Grade 1 . | Grade 2 . | |
| Rash/desquamation | 4 (9.8%) | 4 (9.8%) | |
| Nausea | 17 (41.5%) | 3 (7.3%) | |
| Vomiting | 15 (36.6%) | 6 (14.6%) | |
| SGOT (AST) | 8 (19.5%) | 3 (7.3%) | |
| SGPT (ALT) | 6 (14.6%) | 5 (12.2%) | |
| Headache | 3 (7.3%) | 7 (17.1%) | |
| . | Maximum Grade across the Course . | . | |
|---|---|---|---|
| Toxicity Type . | Grade 1 . | Grade 2 . | |
| Rash/desquamation | 4 (9.8%) | 4 (9.8%) | |
| Nausea | 17 (41.5%) | 3 (7.3%) | |
| Vomiting | 15 (36.6%) | 6 (14.6%) | |
| SGOT (AST) | 8 (19.5%) | 3 (7.3%) | |
| SGPT (ALT) | 6 (14.6%) | 5 (12.2%) | |
| Headache | 3 (7.3%) | 7 (17.1%) | |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; AST, aspartate aminotransferase; SGPT, serum glutamic pyruvic transaminase; ALT, alanine aminotransferase
Number of patients (%) with non–dose-limiting non-hematologic toxicities related to protocol therapy and observed in more than 10% of 41 evaluable patients
| . | Maximum Grade across the Course . | . | |
|---|---|---|---|
| Toxicity Type . | Grade 1 . | Grade 2 . | |
| Rash/desquamation | 4 (9.8%) | 4 (9.8%) | |
| Nausea | 17 (41.5%) | 3 (7.3%) | |
| Vomiting | 15 (36.6%) | 6 (14.6%) | |
| SGOT (AST) | 8 (19.5%) | 3 (7.3%) | |
| SGPT (ALT) | 6 (14.6%) | 5 (12.2%) | |
| Headache | 3 (7.3%) | 7 (17.1%) | |
| . | Maximum Grade across the Course . | . | |
|---|---|---|---|
| Toxicity Type . | Grade 1 . | Grade 2 . | |
| Rash/desquamation | 4 (9.8%) | 4 (9.8%) | |
| Nausea | 17 (41.5%) | 3 (7.3%) | |
| Vomiting | 15 (36.6%) | 6 (14.6%) | |
| SGOT (AST) | 8 (19.5%) | 3 (7.3%) | |
| SGPT (ALT) | 6 (14.6%) | 5 (12.2%) | |
| Headache | 3 (7.3%) | 7 (17.1%) | |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; AST, aspartate aminotransferase; SGPT, serum glutamic pyruvic transaminase; ALT, alanine aminotransferase
Pharmacokinetic Results
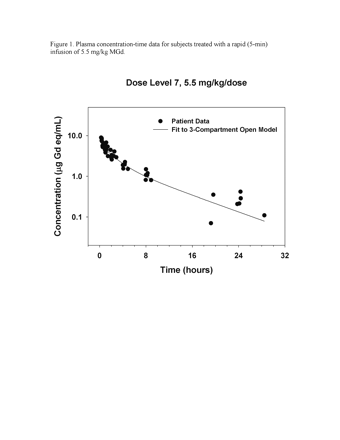
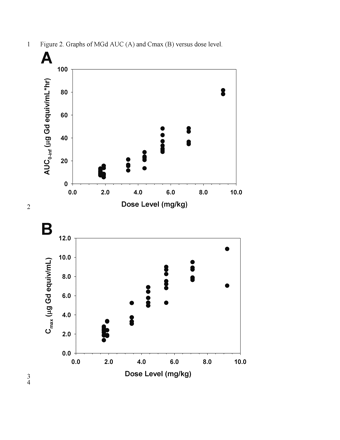
Representative plasma concentration-time data are illustrated in Figure 1. Area under the curve (AUC) and maximum measured plasma concentration (Cmax) values appeared to increase in proportion to dose, with the exception of two patients treated with 9.2 mg/kg MGd who appeared to have a greater than proportional increase in AUC (Fig. 2). Noncompartmental analysis found the median elimination half-life and clearance to be 6.6 h (range, 2.8–11.5) and 25.4 ml/kg/h (range, 11.7–45.3), respectively (Table 4). The median fraction of administered dose excreted in urine was 24.5% (range, 2.1%–54.3%).
Summary of pharmacokinetic data
| Level . | Dose (mg/kg) . | n . | . | Half-life (h) . | Cmax (μg/ml) . | AUC0-∞ (μg/ml/h) . | CL (ml/kg/h) . | Vss (ml/kg) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.7 | 4 | Median | 7.1 | 2.3 | 10.7 | 22.3 | 183 |
| Range | 6.4–9.3 | 1.9–2.6 | 7.6–13.3 | 17.5–30.9 | 136–217 | |||
| 2 | 1.7 | 4 | Median | 10.4 | 2.3 | 10 | 22.9 | 237 |
| Range | 10.0–11.5 | 1.4–2.8 | 8.0–10.9 | 21.3–28.4 | 212–297 | |||
| 3 | 1.7 | 4 | Median | 6.3 | 2.4 | 8.7 | 26.8 | 182 |
| Range | 5.8–6.8 | 1.9–2.4 | 7.3–10.2 | 23.0–31.8 | 165–198 | |||
| 4 | 1.9 | 5 | Median | 5.6 | 2.4 | 8.5 | 30.5 | 175 |
| Range | 4.5–7.2 | 1.8–3.4 | 5.7–15.7 | 16.7–45.1 | 131–227 | |||
| 5 | 3.4 | 4 | Median | 5.8 | 3.5 | 16.1 | 29.0 | 157 |
| Range | 5.3–6.5 | 3.1–5.2 | 11.7–21.2 | 22.1–40.0 | 151–228 | |||
| 6 | 4.4 | 5 | Median | 6.6 | 5.8 | 22.6 | 26.5 | 181 |
| Range | 5.5–6.9 | 5.0–6.9 | 13.5–27.5 | 21.9–45.3 | 167–311 | |||
| 7 | 5.5 | 7 | Median | 6.6 | 7.5 | 33.3 | 22.6 | 166 |
| Range | 2.9–8.8 | 5.3–9.0 | 27.8–48.2 | 15.5–27.0 | 38–207 | |||
| 8 | 7.1 | 6 | Median | 6.1 | 8.3 | 36.7 | 26.5 | 181 |
| Range | 5.8–8.5 | 7.6–9.5 | 34.7–48.4 | 20.1–28.1 | 144–220 | |||
| 9 | 9.2 | 2 | Median | 9.1 | 9.0 | 80 | 13.9 | 164 |
| Range | 8.1–10.2 | 7.0–10.9 | 78.3–81.6 | 11.7–16.1 | 116–212 |
| Level . | Dose (mg/kg) . | n . | . | Half-life (h) . | Cmax (μg/ml) . | AUC0-∞ (μg/ml/h) . | CL (ml/kg/h) . | Vss (ml/kg) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.7 | 4 | Median | 7.1 | 2.3 | 10.7 | 22.3 | 183 |
| Range | 6.4–9.3 | 1.9–2.6 | 7.6–13.3 | 17.5–30.9 | 136–217 | |||
| 2 | 1.7 | 4 | Median | 10.4 | 2.3 | 10 | 22.9 | 237 |
| Range | 10.0–11.5 | 1.4–2.8 | 8.0–10.9 | 21.3–28.4 | 212–297 | |||
| 3 | 1.7 | 4 | Median | 6.3 | 2.4 | 8.7 | 26.8 | 182 |
| Range | 5.8–6.8 | 1.9–2.4 | 7.3–10.2 | 23.0–31.8 | 165–198 | |||
| 4 | 1.9 | 5 | Median | 5.6 | 2.4 | 8.5 | 30.5 | 175 |
| Range | 4.5–7.2 | 1.8–3.4 | 5.7–15.7 | 16.7–45.1 | 131–227 | |||
| 5 | 3.4 | 4 | Median | 5.8 | 3.5 | 16.1 | 29.0 | 157 |
| Range | 5.3–6.5 | 3.1–5.2 | 11.7–21.2 | 22.1–40.0 | 151–228 | |||
| 6 | 4.4 | 5 | Median | 6.6 | 5.8 | 22.6 | 26.5 | 181 |
| Range | 5.5–6.9 | 5.0–6.9 | 13.5–27.5 | 21.9–45.3 | 167–311 | |||
| 7 | 5.5 | 7 | Median | 6.6 | 7.5 | 33.3 | 22.6 | 166 |
| Range | 2.9–8.8 | 5.3–9.0 | 27.8–48.2 | 15.5–27.0 | 38–207 | |||
| 8 | 7.1 | 6 | Median | 6.1 | 8.3 | 36.7 | 26.5 | 181 |
| Range | 5.8–8.5 | 7.6–9.5 | 34.7–48.4 | 20.1–28.1 | 144–220 | |||
| 9 | 9.2 | 2 | Median | 9.1 | 9.0 | 80 | 13.9 | 164 |
| Range | 8.1–10.2 | 7.0–10.9 | 78.3–81.6 | 11.7–16.1 | 116–212 |
Abbreviations: Cmax, maximum measured plasma concentration; AUC, area under the curve; CL, plasma clearance; Vss, volume of distribution
Summary of pharmacokinetic data
| Level . | Dose (mg/kg) . | n . | . | Half-life (h) . | Cmax (μg/ml) . | AUC0-∞ (μg/ml/h) . | CL (ml/kg/h) . | Vss (ml/kg) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.7 | 4 | Median | 7.1 | 2.3 | 10.7 | 22.3 | 183 |
| Range | 6.4–9.3 | 1.9–2.6 | 7.6–13.3 | 17.5–30.9 | 136–217 | |||
| 2 | 1.7 | 4 | Median | 10.4 | 2.3 | 10 | 22.9 | 237 |
| Range | 10.0–11.5 | 1.4–2.8 | 8.0–10.9 | 21.3–28.4 | 212–297 | |||
| 3 | 1.7 | 4 | Median | 6.3 | 2.4 | 8.7 | 26.8 | 182 |
| Range | 5.8–6.8 | 1.9–2.4 | 7.3–10.2 | 23.0–31.8 | 165–198 | |||
| 4 | 1.9 | 5 | Median | 5.6 | 2.4 | 8.5 | 30.5 | 175 |
| Range | 4.5–7.2 | 1.8–3.4 | 5.7–15.7 | 16.7–45.1 | 131–227 | |||
| 5 | 3.4 | 4 | Median | 5.8 | 3.5 | 16.1 | 29.0 | 157 |
| Range | 5.3–6.5 | 3.1–5.2 | 11.7–21.2 | 22.1–40.0 | 151–228 | |||
| 6 | 4.4 | 5 | Median | 6.6 | 5.8 | 22.6 | 26.5 | 181 |
| Range | 5.5–6.9 | 5.0–6.9 | 13.5–27.5 | 21.9–45.3 | 167–311 | |||
| 7 | 5.5 | 7 | Median | 6.6 | 7.5 | 33.3 | 22.6 | 166 |
| Range | 2.9–8.8 | 5.3–9.0 | 27.8–48.2 | 15.5–27.0 | 38–207 | |||
| 8 | 7.1 | 6 | Median | 6.1 | 8.3 | 36.7 | 26.5 | 181 |
| Range | 5.8–8.5 | 7.6–9.5 | 34.7–48.4 | 20.1–28.1 | 144–220 | |||
| 9 | 9.2 | 2 | Median | 9.1 | 9.0 | 80 | 13.9 | 164 |
| Range | 8.1–10.2 | 7.0–10.9 | 78.3–81.6 | 11.7–16.1 | 116–212 |
| Level . | Dose (mg/kg) . | n . | . | Half-life (h) . | Cmax (μg/ml) . | AUC0-∞ (μg/ml/h) . | CL (ml/kg/h) . | Vss (ml/kg) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.7 | 4 | Median | 7.1 | 2.3 | 10.7 | 22.3 | 183 |
| Range | 6.4–9.3 | 1.9–2.6 | 7.6–13.3 | 17.5–30.9 | 136–217 | |||
| 2 | 1.7 | 4 | Median | 10.4 | 2.3 | 10 | 22.9 | 237 |
| Range | 10.0–11.5 | 1.4–2.8 | 8.0–10.9 | 21.3–28.4 | 212–297 | |||
| 3 | 1.7 | 4 | Median | 6.3 | 2.4 | 8.7 | 26.8 | 182 |
| Range | 5.8–6.8 | 1.9–2.4 | 7.3–10.2 | 23.0–31.8 | 165–198 | |||
| 4 | 1.9 | 5 | Median | 5.6 | 2.4 | 8.5 | 30.5 | 175 |
| Range | 4.5–7.2 | 1.8–3.4 | 5.7–15.7 | 16.7–45.1 | 131–227 | |||
| 5 | 3.4 | 4 | Median | 5.8 | 3.5 | 16.1 | 29.0 | 157 |
| Range | 5.3–6.5 | 3.1–5.2 | 11.7–21.2 | 22.1–40.0 | 151–228 | |||
| 6 | 4.4 | 5 | Median | 6.6 | 5.8 | 22.6 | 26.5 | 181 |
| Range | 5.5–6.9 | 5.0–6.9 | 13.5–27.5 | 21.9–45.3 | 167–311 | |||
| 7 | 5.5 | 7 | Median | 6.6 | 7.5 | 33.3 | 22.6 | 166 |
| Range | 2.9–8.8 | 5.3–9.0 | 27.8–48.2 | 15.5–27.0 | 38–207 | |||
| 8 | 7.1 | 6 | Median | 6.1 | 8.3 | 36.7 | 26.5 | 181 |
| Range | 5.8–8.5 | 7.6–9.5 | 34.7–48.4 | 20.1–28.1 | 144–220 | |||
| 9 | 9.2 | 2 | Median | 9.1 | 9.0 | 80 | 13.9 | 164 |
| Range | 8.1–10.2 | 7.0–10.9 | 78.3–81.6 | 11.7–16.1 | 116–212 |
Abbreviations: Cmax, maximum measured plasma concentration; AUC, area under the curve; CL, plasma clearance; Vss, volume of distribution
MR Analysis Results
Central review of intratumoral MGd uptake on MR films (digital images were not available for most patients) was performed for 33 patients. On the baseline MRI, MGd uptake was categorized as none in 22 cases, minimal in 3, moderate in 2, and intense in 1. In each of the MR scans showing some MGd intratumoral uptake, the uptake was less than 50% tumor volume. At day 30, MGd uptake was none in 14 cases (no change in tumor intensity in seven; T1 signal related to decreased hydration status increased in seven); minimal in two (<50% tumor volume); moderate in nine (<50% tumor volume in eight; >50% tumor volume in one); and intense in four (<50% tumor volume in two; >50% tumor volume in two). Therefore, at baseline, only 21% of scans demonstrated any enhancement, but by day 30, MGd uptake (as analyzed on noncontrast images) was seen in 52% of cases. In two cases, differentiation of MGd uptake from intratumoral hemorrhage could not be determined. Five and four patients, respectively, had MRI scans at baseline and on day 30 that could not be evaluated or were not available.
Plasma concentration-time data for subjects treated with a rapid (5-min) infusion of 5.5 mg/kg motexafin gadolinium (MGd).
Graphs of motexafin gadolinium (MGd) area under the curve (AUC) (A) and maximum measured plasma concentration (Cmax) (B) versus dose level.
Discussion
The combination of the radiosensitizing drug MGd, when administered Monday–Friday at a daily dose of 4.4 mg/kg, with 54 Gy radiation therapy over a 6-week period was relatively well tolerated in children with newly diagnosed brainstem gliomas. The primary toxicities included hypertension and elevation in serum transaminases, a toxicity profile similar to that observed in adult patients.22,23 The MTD in children defined in the present study is similar to the multiday MTD observed in adult patients,22 even though the total cumulative dose administered to children was higher. Systemic exposure appeared proportional to the dose administered. Drug disposition in children is similar to that observed in adult patients,22,24 with the median plasma drug clearance of 25.4 ml/kg/h in children comparable to a mean population model derived clearance (corrected for a 70-kg ideal body weight) of 23.4 ml/kg/h observed in adult patients.25
Preclinical studies have demonstrated that MGd, like naturally occurring porphyrins, selectively accumulates in tumors.21 It enhances radiation-induced apoptosis, possibly via induction of futile redox cycling and depletion of intracellular antioxidants such as glutathione, ascorbate, lipoate, and others. Preclinical pharmacokinetic studies and MRI drug-distribution analyses in SMTF tumor-bearing mice suggest that the 2- to 5-h window following MGd delivery is optimal for radiation therapy.26 Fast-growing spontaneous mammary tumors were transplanted into mice by intramuscular injection. The tumor-bearing animals pretreated with MGd prior to radiation showed enhanced survival and a higher percentage of complete responders compared to animals receiving radiation alone.26
The single dose MTD of MGd in adult subjects was 22.3 mg/kg, with dose-limiting reversible acute renal failure observed at 29.6 mg/kg. In adult patients with newly diagnosed glioblastoma multiforme, the MTD of MGd, when administered daily for the first 10 fractions and 3 times per week thereafter with radiotherapy, was 4.0–5.3 mg/kg/day.27 Two randomized phase III trials comparing whole-brain radiotherapy (30 Gy/10 fractions) with or without MGd (5 mg/kg/day before each fraction) in patients with brain metastases have been completed. The most common grade 3 or 4 adverse events possibly related to MGd were hypertension (5.8%), asthenia (2.6%), hyponatremia (2.1%), leucopenia (2.1%), hyperglycemia (1.6%), and vomiting (1.6%).28,29 A conservative starting dose was chosen for this pediatric study because the MTD from adult studies with MGd was not determined at the time this study was opened. The study was then amended once the adult MTD was established to provide an approximately 80% dose increase between the fourth and fifth dose levels.
In our MR analysis of intratumoral uptake, we found that intrinsic pontine gliomas may decrease their water content, reflected by an increased T1 signal and decreased T2 signal, with therapy. This phenomenon can also be observed with administration of corticosteroids,30 which was permitted in the current study to treat increased intracranial pressure. Differentiation of MGd uptake from subacute hemorrhage, however, can be difficult, as both will display increased signal on T1-weighted images. The addition of axial gradient echo susceptibility T2-weighted sequences to imaging protocols in future MGd studies may help to differentiate MGD uptake from subacute hemorrhage. By day 30, more than half the tumors had MGd uptake. However, whether this represents drug penetration due to disruption of the blood-brain barrier from radiotherapy or a cumulative drug effect is not known.
In summary, the MTD of MGd in combination with standard, once-daily radiation therapy in pediatric patients with newly diagnosed diffuse pontine gliomas is 4.4 mg/kg/day, delivered as a 5-min bolus 2–5 h prior to each radiation fraction. As local relapse remains the dominant failure pattern in intrinsic pontine glioma, it is important to continue to investigate new agents and strategies to improve local control and outcome in this disease. In this study, although only three cases demonstrated greater than 50% MGd-to-tumor volume ratio at day 30, over half of the patients demonstrated some MGd uptake at day 30, suggesting that the drug is taken up in tumor. Tumor uptake of drug is critical for agents whose mechanism of action is to provide radiosensitization. Further analysis of the correlation of tumor uptake to local control and outcome will be performed in the COG phase II study.
This research was supported by COG grant CA 98543 and COG Phase I CA 97452. A complete listing of grant support for research conducted by the Children's Cancer Group and Pediatric Oncology Group before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm.
References
Smith MA, Freidlin B, Ries LA, et al. Trends in reported incidence of primary malignant brain tumors in children in the United States.
Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas.
Albright AL, Guthkelch AN, Packer RJ, et al. Prognostic factors in pediatric brain-stem gliomas.
Fisher PG, Breiter SN, Carson BS, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities.
Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review.
Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study.
Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials.
Lewis J, Lucraft H, Gholkar A. UKCCSG study of accelerated radiotherapy for pediatric brain stem gliomas. United Kingdom Childhood Cancer Study Group.
Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial.
Prados MD, Wara WM, Edwards MS, et al. The treatment of brain stem and thalamic gliomas with 78 Gy of hyperfractionated radiation therapy.
Rubin G, Michowitz S, Horev G, et al. Pediatric brain stem gliomas: an update.
Fisher PG, Donaldson SS. Hyperfractionated radiotherapy in the management of diffuse intrinsic brainstem tumors: when is enough enough?
Packer RJ, Allen JC, Goldwein JL, et al. Hyperfractionated radiotherapy for children with brainstem gliomas: a pilot study using 7,200 cGy.
Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Children's Cancer Group Phase I/II Trial.
Bernier-Chastagner V, Grill J, Doz F, et al. Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas: results of a French Society of Paediatric Oncology Phase II Study.
Doz F, Neuenschwander S, Bouffet E, et al. Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Société Française d'Oncologie Pédiatrique.
Marcus KJ, Dutton SC, Barnes P, et al. A phase I trial of etanidazole and hyperfractionated radiotherapy in children with diffuse brainstem glioma.
Packer RJ, Krailo M, Mehta M, et al. Phase 1 study of concurrent RMP-7 and carboplatin with radiotherapy for children with newly diagnosed brainstem gliomas.
Sanghavi SN, Needle MN, Krailo MD, et al. A phase I study of topotecan as a radiosensitizer for brainstem glioma of childhood: first report of the Children's Cancer Group-0952.
Hashemy SI, Ungerstedt JS, Zahedi Avval F, et al. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase.
Carde P, Timmerman R, Mehta MP, et al. Multicenter phase Ib/II trial of the radiation enhancer motexafin gadolinium in patients with brain metastases.
Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points.
Ramanathan RK, Fakih M, Mani S, et al. Phase I and pharmacokinetic study of the novel redox-active agent, motexafin gadolinium, with concurrent radiation therapy in patients with locally advanced pancreatic or biliary cancers.
Miles DR, Smith JA, Phan SC, et al. Population pharmacokinetics of motexafin gadolinium in adults with brain metastases or glioblastoma multiforme.
Miller RA, Woodburn K, Fan Q, et al. In vivo animal studies with gadolinium (III) texaphyrin as a radiation enhancer.
Mehta M, Ford JM, Suh J, et al. Cumulative dose of motexafin gadolinium and survival in newly diagnosed glioblastoma multiforme [abstract].
Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial.
Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases.