-
PDF
- Split View
-
Views
-
Cite
Cite
Karst Y Heida, Michiel L Bots, Christianne JM de Groot, Frederique M van Dunné, Nurah M Hammoud, Annemiek Hoek, Joop SE Laven, Angela HEM Maas, Jeanine E Roeters van Lennep, Birgitta K Velthuis, Arie Franx, Cardiovascular risk management after reproductive and pregnancy-related disorders: A Dutch multidisciplinary evidence-based guideline, European Journal of Preventive Cardiology, Volume 23, Issue 17, 1 November 2016, Pages 1863–1879, https://doi.org/10.1177/2047487316659573
Close - Share Icon Share
Abstract
In the past decades evidence has accumulated that women with reproductive and pregnancy-related disorders are at increased risk of developing cardiovascular disease (CVD) in the future. Up to now there is no standardised follow-up of these women becausee guidelines on cardiovascular risk management for this group are lacking. However, early identification of high-risk populations followed by prevention and treatment of CVD risk factors has the potential to reduce CVD incidence. Therefore, the Dutch Society of Obstetrics and Gynaecology initiated a multidisciplinary working group to develop a guideline for cardiovascular risk management after reproductive and pregnancy-related disorders.
The guideline addresses the cardiovascular risk consequences of gestational hypertension, preeclampsia, preterm delivery, small-for-gestational-age infant, recurrent miscarriage, polycystic ovary syndrome and premature ovarian insufficiency. The best available evidence on these topics was captured by systematic review. Recommendations for clinical practice were formulated based on the evidence and consensus of expert opinion. The Dutch societies of gynaecologists, cardiologists, vascular internists, radiologists and general practitioners reviewed the guideline to ensure support for implementation in clinical practice.
For all reproductive and pregnancy-related disorders a moderate increased relative risk was found for overall CVD, except for preeclampsia (relative risk 2.15, 95% confidence interval 1.76–2.61).
Based on the current available evidence, follow-up is only recommended for women with a history of preeclampsia. For all reproductive and pregnancy-related disorders optimisation of modifiable cardiovascular risk factors is recommended to reduce the risk of future CVD.
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in women.1 Although CVD deaths have declined considerably in the past decades worldwide they still account for 17.3 million deaths annually.2 Early identification of high-risk populations followed by prevention and treatment of cardiovascular risk factors has the potential to reduce CVD incidence.3 In the last decade it became increasingly clear that several disorders of pregnancy and reproduction are related to an increased risk of future CVD morbidity and mortality.4,5 This resulted in the concept that pregnancy may be considered a cardiovascular ‘stress test’ for women, which some do not pass (i.e. they develop a pregnancy-related disorder).6 In the literature, the reproductive disorders polycystic ovary syndrome (PCOS) and premature ovarian insufficiency (POI) have also been related to an increased CVD risk.7,8 Thus information on reproductive and pregnancy-related disorders has the potential to identify women at increased CVD risk, who could potentially benefit from early preventive strategies. Moreover, women with reproductive and pregnancy-related disorders are diagnosed at a young age and consequently it takes la ong time to develop CVD events.
In daily practice, however, neither women with a reproductive or pregnancy-related disorder nor their treating physicians are aware of the potentially increased CVD risk.9,10 There are no clinical guidelines on how to manage postpartum CVD prevention after reproductive and pregnancy-related disorders, and as a consequence follow-up is rarely offered to these women. In the past few years, several recommendations have been published on cardiovascular risk management after hypertensive disorders of pregnancy (HDP).11,12 However, these recommendations were not based on systematic review of the available evidence and consensus between the various multidisciplinary healthcare professionals involved in the follow-up of women with a reproductive or pregnancy-related disorder. Moreover, the lack of a national guideline on this topic is likely to lead to undesired practice variation and to both under and over treatment with a potential waste of resources in healthcare.
Recently, the American Heart Association (AHA) included pregnancy-related disorders, preeclampsia, gestational diabetes and gestational hypertension as risk factors for CVD in their latest guideline on the prevention of cardiovascular disease in women.13 It was recommended that healthcare professionals should obtain pregnancy history as part of routine clinical intake. However, in clinical practice this recommendation is not always implemented. In the 2014 guidelines for the prevention of stroke in women the AHA recommends that women with preeclampsia should be evaluated 6 months to 1 year postpartum to assess and potentially treat cardiovascular risk factors including hypertension, obesity, smoking and dyslipidaemia.14 In this guideline, however, there was only a focus on preeclampsia instead of all reproductive and pregnancy-related disorders.
In 2012 the Dutch Society of Obstetrics and Gynecology initiated a multidisciplinary development group to develop a guideline on cardiovascular risk management after the following disorders: preeclampsia, gestational hypertension, preterm delivery (PTD), small-for-gestational-age (SGA) infant, recurrent miscarriage, PCOS and POI. These disorders have been associated in the literature with increased prevalence of CVD risks factors and/or events. Gestational diabetes was not included because follow-up after this disorder has been described in another Dutch guideline. The Dutch guideline was completed in the second half of 2014. It contains literature reviews and meta-analyses on associations of reproductive and pregnancy-related disorders with cardiovascular event risk and risk factors as well as recommendations for follow-up of these disorders. This paper describes the summary of this guideline.
Methods
The methods can be found in supplementary section 1.
Results
Hypertensive disorders of pregnancy
Background
HDP contribute substantially to maternal and neonatal morbidity and mortality,15 and are associated with CVD of the mother later in life.4 HDP can be divided into gestational hypertension (GH) and preeclampsia. Different definitions are used in the literature. The International Society for the Study of Hypertension in Pregnancy criteria have been adopted in The Netherlands.16 According to these criteria, GH is defined as a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg, measured on at least two occasions after 20 weeks of gestation. Preeclampsia is defined as the same pattern of elevated blood pressure but with proteinuria (≥300 mg/24 hour). Preeclampsia can be distinguished into an early (<34 weeks of gestation) and a late onset (>37 weeks of gestation).
Reported incidences of HDP vary strongly and depend on definitions used and populations studied. Of all pregnancies in The Netherlands the incidence of GH is approximately 15%, and is 2% for preeclampsia.17 Since the pathophysiological pathways leading to GH and early or late onset preeclampsia might be (partly) different this could lead to different relations between CVD and GH or preeclampsia.18 Therefore we report these conditions separately when possible.
Summary of the literature
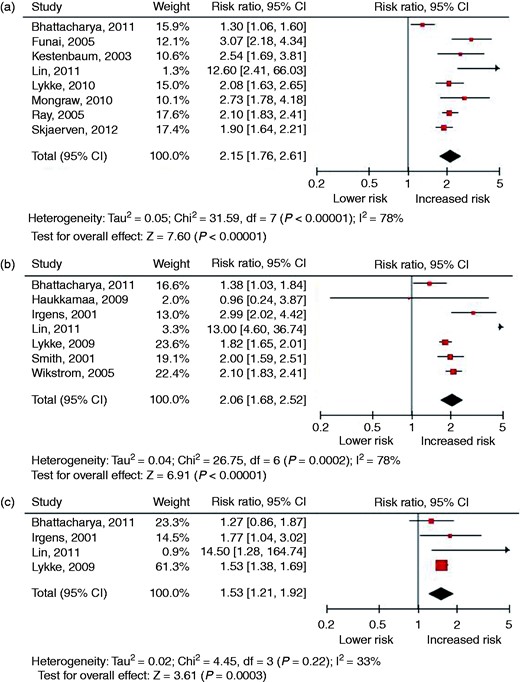
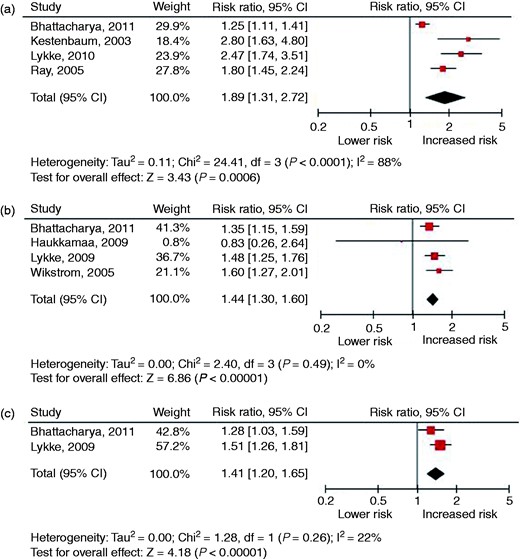
Twenty-eight cohort studies and 20 case–control studies were identified. The sample sizes of the individual studies ranged from 40 to 1,030,000 and follow-up ranged from 3 to 37 years. Figures 1 and 2 present the forest plots of studies investigating preeclampsia and GH in relation to the risk of fatal and non-fatal CVD and supplementary Tables 2 and 3 present the results of the meta-analysis from studies that have examined the relation between preeclampsia and GH and the risk of CVD (risk factors). Preeclampsia was related to an increased risk of developing or dying from overall CVD (relative risk (RR) 2.15, 95% confidence interval (CI) 1.76–2.61), ischaemic heart disease (IHD) (RR 2.06, 95% CI 1.68–2.52) and stroke (RR 1.53, 95% CI 1.21–1.92). The risk of developing hypertension (RR 2.76, 95% CI 1.63–4.69) and diabetes (RR 2.27, 95% CI 1.55–3.32) was also increased compared to women with a normotensive pregnancy. GH was also related to an increased risk of developing or dying from overall CVD (RR 1.89, 95% CI 1.31–2.72), IHD (RR 1.44, 95% CI 1.30–1.60) and stroke (RR 1.41, 95% CI 1.20–1.65). The risk of developing hypertension (RR 2.87, 95% CI 0.84–9.77) was not increased compared to women with a normotensive pregnancy, although this risk was based on two cohort studies.
Forest plots of studies investigating a history of preeclampsia in relation to the risk of fatal and non-fatal cardiovascular disease: (a) overall cardiovascular disease; (b) ischaemic heart disease; (c) stroke.
Forest plots of studies investigating a history of gestational hypertension in relation to the risk of fatal and non-fatal cardiovascular disease: (a) overall cardiovascular disease; (b) ischaemic heart disease; (c) stroke.
We found four studies in which early and late onset preeclampsia was separated.19–22 Mongraw et al. defined early onset preeclampsia as an onset before 34 weeks of gestation and reported a RR of 9.5 (95% CI 3.5–20.3) of developing a cardiovascular event.21 Skjaerven et al. defined early onset preeclampsia when it occurred before 36 weeks of gestation. They found a RR of 3.7 (95% CI 2.7–4.8) of developing a cardiovascular event.20 Similar ranges were found for stroke and IHD in two other studies in which early onset preeclampsia was defined as an onset before 36 and 37 weeks of gestation, respectively.19,22
Considerations
Studies published until the end of 2012 consistently showed that a history of preeclampsia is associated with a significantly increased RR of a cardiovascular event in the future, particularly in women with early onset preeclampsia. These findings are comparable with the results of other meta-analysis such as Bellamy et al. with a RR of 2.16 to develop IHD and 1.81 to develop stroke.4 However, it should be noted that in our analysis more recent studies were included and that the results of the most complete multivariate models with adjustment for potential confounders were used. The associations we found for GH appeared to be weaker, although still present. Also, both preeclampsia and GH were positively associated with the presence or development of the cardiovascular risk factors hypertension and diabetes. No relation with dyslipidaemia later in life was observed. This may be related to the relatively young age of the included predominantly premenopausal women.
Between-study heterogeneity should also be taken into account, because the included studies had variable follow-up durations and adjustment for different confounders. The heterogeneity in results among studies was evaluated by I2 statistics. This is a measure of inconsistency that describes the percentage of observed variability in results, which reflects real differences in effects rather than variation that can be expected due to chance. Values of I2 > 50% indicate significant heterogeneity.23 Although most meta-analyses had an I2 > 50%, all included studies showed a positive effect on the relation between CVD and preeclampsia/GH.
An important knowledge gap is the question of whether preeclampsia/GH are independent risk factors for CVD or if they act solely via traditional CVD risk factors. It is clear that women with preeclampsia/GH are at increased risk of developing hypertension later in life. Hypertension itself is a risk factor for CVD.24 Unfortunately, hypertension is not included as a confounder in most studies. Garovic et al. made adjustment for hypertension and diabetes in their analysis and did not find a significantly increased risk for IHD (hazard ratio (HR) 1.14, 95% CI 0.78–1.68), although HDP was significantly associated with stroke (HR 1.86, 95% CI 1.16–2.98).25 However, these results were based on a relatively small study population with 4720 included women and the outcome was based on non-fatal events only.
Recommendations for cardiovascular risk management in women with a history of preeclampsia
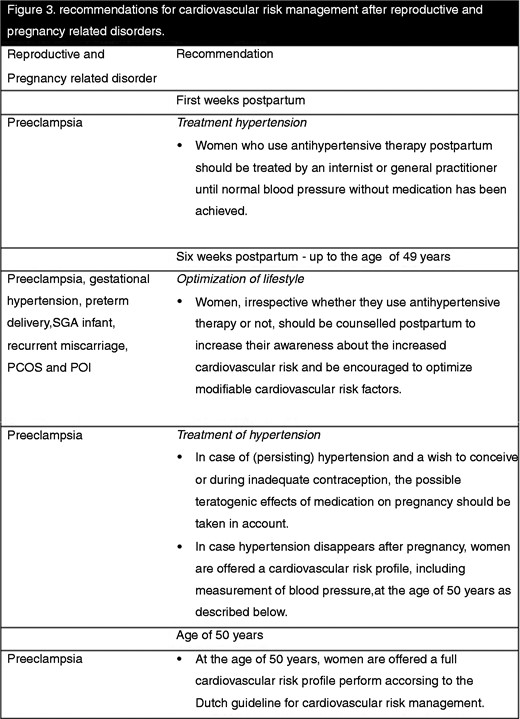
There is only limited support in the literature for specific diagnostic and treatment strategies. The associations between reproductive and pregnancy-related disorders on the one hand and CVD on the other have been widely studied. However, there are no randomised controlled trials (RCTs) evaluating interventions to reduce CVD risk in women with pregnancy-related disorders. Nevertheless, based on the number and quality of the studies and the consistent RR above the (arbitrary) cut-off 2 to develop CVD (level B evidence), the guideline development group recommends that a follow-up for women with a history of preeclampsia should be established, as shown in Figure 3.
Recommendation for cardiovascular risk management after reproductive and pregnancy-related disorders.
The goal of encouraging these women to optimise modifiable cardiovascular risk factors is to create awareness among women with a history of preeclampsia to change their lifestyle. Lifestyle interventions during reproductive age have potentially a large effect on future cardiovascular health. The benefit of ideal cardiovascular health is evident from large observational studies.26,27 Moreover, lifestyle interventions after preeclampsia have the potential to decrease cardiovascular risk by 4–13%.28
If antihypertensive medication is prescribed, first choice treatment for these women is β blockers or dihydropyridine calcium antagonists. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and renin inhibitors are contraindicated in pregnancy because of teratogenicity.29
The choice for a cardiovascular risk profile at the age of 50 years is a pragmatic one and was obtained by consensus between the different participating scientific societies. The ideal age of screening of cardiovascular risk factors in women with preeclampsia should be studied further. For optimal implementation in clinical practice in The Netherlands, this guideline aligns with the Dutch guideline for cardiovascular risk management summarised in supplementary section 3.
Recommendations for cardiovascular risk management in women with a history of GH
The RR of developing hypertension is approximately 2 and the RR of developing CVD is lower than 2 in women with GH (level B evidence). However, the number and quality of available studies are limited. Therefore, the guideline development group recommends no specific follow-up for these women. Nevertheless, the development group recommends that women with a history of GH should be encouraged to optimise modifiable cardiovascular risk factors to reduce the risk of future CVD.
Spontaneous Preterm Delivery
Background
PTD is the most common cause of neonatal morbidity and mortality worldwide.30 Recent literature also relates it to an increased risk of CVD for the mother.31,32 PTD is defined as a delivery before 37 weeks of gestation. In the USA, the rate of infants born before 37 weeks of gestation is 12–13%; while in Europe and other developed countries these rates vary between 5% and 11%.33,34 About 30–35% of preterm births are medically indicated.35 The main reasons for these medically indicated deliveries include preeclampsia and fetal growth restriction,36 which have both also been associated with maternal CVD risk.4,37 A combination of these pregnancy-related disorders is associated with an even higher risk of developing CVD.20,37 Approximately 70% of all PTD are the result of spontaneous labour or preterm prelabour rupture of the membranes. In these cases the aetiology remains unknown most of the time, but some of the spontaneous PTD seem to result from infection, uterine overdistension or uterine anomalies. For the purpose of this guideline, only studies that assessed spontaneous PTD were selected, because the subjects with HDP and SGA infant are discussed separately in this guideline.
Summary of the literature
Recently, the results of the meta-analysis on the relation between CVD and spontaneous PTD were published in a more comprehensive review of the same authors as this guideline.38
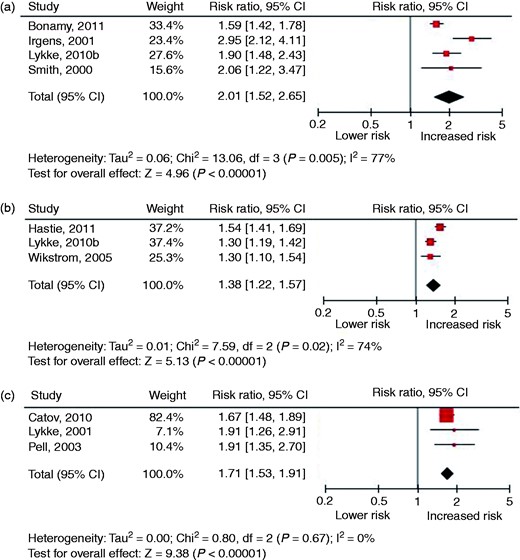
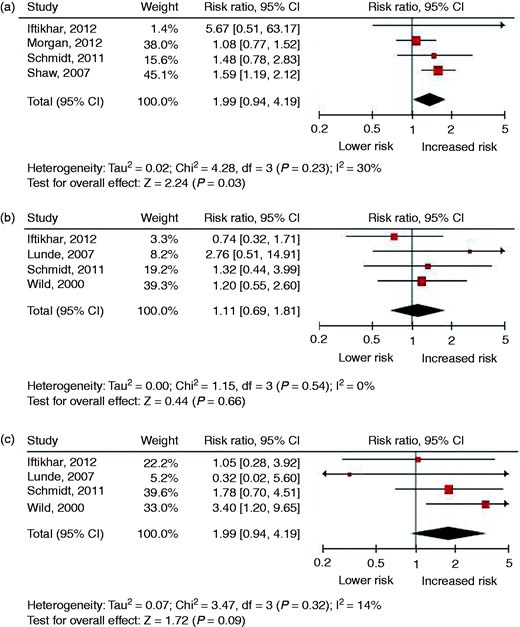
Eleven cohort studies were identified. The sample sizes of the individual studies ranged from 446 to 923,686 and follow-up ranged from 12 to 35 years. In all studies cases of preeclampsia and/or SGA infant were excluded or adjustment was made for the confounders preeclampsia and SGA infant. Therefore, the assumption was made that the results of this meta-analysis represent the relation between spontaneous PTD and CVD. Figure 4 presents the forest plots of studies investigating spontaneous PTD in relation to the risk of fatal and non-fatal CVD and Supplementary Table 4 presents the results of the meta-analysis from studies that have examined the relation between spontaneous PTD and the risk of CVD (risk factors). Spontaneous PTD was related to an increased risk of developing or dying from overall CVD (RR 2.06, 95% CI 1.58–2.68), IHD (RR 1.38, 95% CI 1.22–1.57) and stroke (RR 1.71, 95% CI 1.53–1.91). The risk of developing hypertension (RR 1.29, 95% CI 1.24–1.35) and diabetes (RR 1.77, 95% CI 1.46–2.15) was also increased compared to women with a term delivery.
Considerations
The meta-analysis showed that women with a history of PTD have a two-fold increased risk of CVD later in life. Although between-study heterogeneity was substantial, all studies showed a positive effect on the relation between CVD and PTD. The between-study heterogeneity might be an effect of the small number of studies, different outcome measures, use of confounders and duration of follow-up.
Most studies adjusted for socioeconomic status, although the results of these studies did not differ from those without adjustment for socioeconomic status. Smoking, associated with both an increased risk of PTD and CVD,39,40 was not measured in most studies. This may have led to overestimation of the associations. Only one study adjusted for antenatal smoking, and although the association modestly diminished after correction, the reported risk was still comparable with the other studies.37
Recommendations for cardiovascular risk management in women with a history of spontaneous PTD
The RR of developing CVD is above 2 in women with a history of PTD (level B evidence). However, the number of studies available is limited and there is an omission of adjustment for smoking, an important confounder for both PTD and CVD. Therefore, the guideline development group recommends no specific follow-up for these women. Nevertheless, the development group recommends that women with a history of a PTD should be encouraged to optimise modifiable cardiovascular risk factors to reduce the risk of future CVD.
Small-for-Gestational-Age infant
Background
SGA is defined as a birth weight below the 10th percentile of the reference population. This corresponds with a birth weight smaller than 2 standard deviations (SD) below the mean. In most cases impaired fetal growth is a consequence of an insufficient utero–placental circulation. This utero–placental insufficiency is associated with gestational hypertension or preeclampsia, but can also occur independently of these HDP. In a subsequent pregnancy, women with a previous SGA infant have an increased risk of recurrent fetal growth restriction. This holds for both women with and without hypertension in their first pregnancy.41,42 Fetal growth restriction is associated with increased risk of perinatal morbidity and mortality, and with chronic diseases in adult life, such as CVD, diabetes and hypertension.43 In addition to these risks for the child, delivering a SGA infant appears to increase the risk for the mother to develop cardiovascular morbidity and mortality.
Summary of the literature
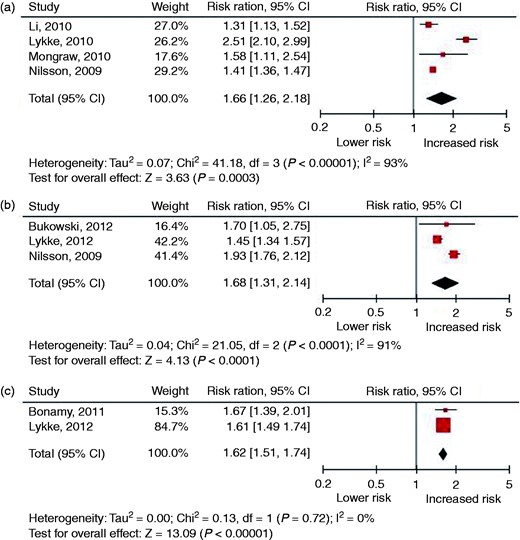
Nine studies were identified. The sample sizes of the individual studies ranged from 6608 to 1,400,000 and follow-up ranged from 11.8 to 37 years. Figure 5 presents the forest plots of studies investigating giving birth to a SGA infant in relation to the risk of fatal and non-fatal CVD and Supplementary Table 5 shows the results of the meta-analysis from studies that have examined the relation between giving birth to a SGA infant and the risk of CVD (risk factors). Delivery of a SGA infant was related to an increased risk of developing or dying from overall CVD (RR 1.66, 95% CI 1.26–2.18), IHD (RR 1.68, 95% CI 1.31–2.14) and stroke (RR 1.62, 95% CI 1.51–1.74). As only one cohort study reported on the relation of SGA with hypertension and diabetes,44 we could not perform a meta-analysis for these risk factors.
Forest plots of studies investigating a history of preterm delivery in relation to the risk of fatal and non-fatal cardiovascular disease: (a) overall cardiovascular disease; (b) ischaemic heart disease; (c) stroke.
Nilson et al. showed that the risk of CVD increases in a stepwise manner with the number of SGA infants.45 Delivering one SGA infant was related to an increased risk of 1.41 (95% CI 1.36–1.46), two SGA infants with a RR of 1.74 (95% CI 1.58–1.93) and three or more SGA infants with a RR of 1.86 (95% CI 1.35–2.57).
Another study differentiated between moderate (birth weight between 2 and 3 SD below the mean) and severe (birth weight smaller than 3 SD below the mean) SGA.44 In the severe SGA group they found a higher risk for developing IHD (RR 1.84 (95% CI 1.55–2.18) versus RR 1.35 (95% CI 1.23–1.48)) and stroke (RR 1.91 (95% CI 1.62–2.25) versus RR 1.53 (95% CI 1.40–1.67)).
Considerations
Although all studies found a positive effect on the relation between CVD and a history of a SGA infant, the overall risk was only moderately increased. Again substantial between-study heterogeneity was found, probably the effect of the small number of studies. The included studies used different definitions of SGA. In most American studies a birth weight below 2500 g was used to define SGA. This corresponds with a birth weight <10th percentile after 37 weeks of gestation for the US population.46 Generally, the reference group consisted of (only) appropriate-for-gestational-age infants. A few studies did not specify whether large-for-gestational-age children were included in the control group. However, we do not expect that this would have led to underestimation of the associations, because mothers of large-for-gestational-age infants are not at increased risk of CVD.47,48
Smoking is not only associated with the risk of giving birth to a SGA infant,49 but also with an increased risk of CVD50 and is thus a potential confounder. Only two studies adjusted for smoking.48,51
SGA is common in preeclamptic pregnancies, with an incidence of 12%, especially in early-onset preeclampsia.52 A combination of SGA and preeclampsia is associated with an even higher risk of dying from a cardiovascular cause (HR 4.67, 95% CI 2.89–7.55).53 For women with a history of preeclampsia follow-up is recommended as described in Supplementary section 4.1.4.
Recommendations for cardiovascular risk management in women with a history of a SGA infant
The RR of developing CVD is lower than 2 in women with a history of a SGA infant (level B evidence). Therefore, the guideline development group recommends no specific follow-up for these women. Nevertheless, the development group recommends that women with a history of a SGA infant should be encouraged to optimise modifiable cardiovascular risk factors to reduce the risk of future CVD.
Recurrent miscarriage
Background
Miscarriage is a very common complication of pregnancy. In each pregnancy the risk of a miscarriage ranges from 12% to 15%. This risk increases with the age of the woman, up to >50% at an age of 42 years.54 The World Health Organization defines miscarriage as the premature loss of a fetus up to 23 weeks of pregnancy and weighing up to 500 g. However in The Netherlands the term miscarriage is used when a pregnancy ends before a gestational age of 16 weeks counting from the first day of the last menstrual period.
Internationally recurrent miscarriage is defined as three consecutive miscarriages, although in the literature the cut-off of two or more miscarriages is also commonly used. The incidence of recurrent miscarriage is about 3% with the definition of two consecutive miscarriages and 1% with three consecutive miscarriages.55 The cause of (recurrent) miscarriages is heterogeneous. One hypothesis is that aberrant vascularisation of the trophoblast/placenta leads to miscarriage. These vascular abnormalities, related to inherited and acquired thrombophilias, are both associated with miscarriages56 and CVD.57 Other joint risk factors for CVD and miscarriage are obesity,58 smoking and alcohol intake.59 However, long-term cardiovascular implications of recurrent miscarriage are not well known. In this section we assessed the relation between recurrent miscarriage and CVD.
Summary of the literature
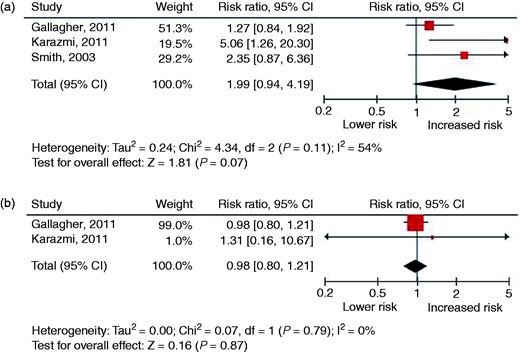
Three cohort studies and two case–control studies were identified. The sample sizes of the individual studies ranged from 123 to 267,400 and follow-up ranged from 11 to 19 years. Figure 6 presents the forest plots of studies investigating recurrent miscarriage in relation to the risk of fatal and non-fatal CVD and Supplementary Table 6 shows the results of the meta-analysis from studies that have examined the relation between recurrent miscarriage and the risk of CVD (risk factors). No relation was found between recurrent miscarriage and the risk of developing or dying from IHD (RR 1.99, 95% CI 0.94–4.19) or stroke (RR 0.98, 95% CI 0.80–1.21). We did not find studies that reported on overall CVD or risk factors and recurrent miscarriage.
Forest plots of studies investigating a history of a small-for-gestational-age infant in relation to the risk of fatal and non-fatal cardiovascular disease: (a) overall cardiovascular disease; (b) ischaemic heart disease; (c) stroke.
Considerations
Although all cohort studies showed a positive association between recurrent miscarriage and IHD, the pooled risk did not reach statistical significance. This may be due to low numbers of incident IHD cases resulting in wide confidence intervals, despite pooling of data. Kharazami et al. found the strongest relation between three or more miscarriages and myocardial infarction later in life (HR 8.90, 95% CI 3.18–24.90), although this relation was attenuated by adjustment for CVD risk factors, including smoking, alcohol consumption, body mass index (BMI), hypertension, hyperlipidaemia and diabetes mellitus (HR 5.06, 95% CI 1.26–20.29).60 No relation was found between recurrent miscarriage and stroke.
From the few studies that assessed the relation between recurrent miscarriage and CVD, it was not clear whether women had thrombophilia, hyperhomocysteinaemia or another identifiable cause for recurrent miscarriage. These disorders are might be associated with CVD and if so, this common pathway might explain the relation between recurrent miscarriage and CVD. Another relevant aspect is the use of different definitions for recurrent miscarriage, which leads to heterogeneity in results.
Recommendations for cardiovascular risk management in women with a history of recurrent miscarriage
The RR of developing CVD is lower than 2 in women with a history of recurrent miscarriage (level B evidence). Therefore, the guideline development group recommends no specific follow-up for these women. Nevertheless, the development group recommends that women with a history of recurrent miscarriage should be encouraged to optimise modifiable cardiovascular risk factors to reduce the risk of future CVD.
Polycystic ovary syndrome
Background
PCOS is the most common endocrine disorder in women of reproductive age affecting around 5–10% of women.61 PCOS is a heterogeneous reproductive disorder, which is diagnosed when at least two out of the following three criteria are present: oligo or anovulation, clinical or biochemical signs of hyperandrogenism and polycystic ovaries on ultrasound.62 Next to cycle abnormality and infertility, both obese and non-obese women with PCOS are also prone to metabolic abnormalities such as glucose intolerance, dyslipidaemia, hypertension and metabolic syndrome.63,64 Women with PCOS are at substantially increased risk of developing type 2 diabetes. Nevertheless, the literature on this association between PCOS and CVD is scarce and contradictory.8 This has been attributed to the retrospective design of most published studies, with the usual uncertainties on initial diagnosis, the length of follow-up and loss to follow-up, small sample size, characteristics of the control group, and accuracy of events diagnosed at later ages. In this section we attempted a meta-analysis to determine the relation between PCOS and CVD (risk factors).
Summary of the literature
Seven cohort studies, five case–control studies and one meta-analysis were identified. The sample sizes of the individual studies ranged from 127 to 108,676 and follow-up ranged from 4.7 to 31 years. Figure 7 presents the forest plots of studies investigating PCOS in relation to the risk of fatal and non-fatal CVD and Supplementary Table 7 presents the results of the meta-analysis from studies that have examined the relation between PCOS and the risk of CVD (risk factors). PCOS was related to an increased risk of developing or dying from overall CVD (RR 1.38, 95% CI 1.04–1.83), but no relation was found for IHD (RR 1.11, 95% CI 0.69–1.81) and stroke (RR 1.79, 95% CI 0.92–3.48). The strongest relation was found for type 2 diabetes. In the meta-analysis of Moran et al. women with PCOS appeared to be at higher risk for type 2 diabetes than women without PCOS (odds ratio (OR) 4.43, 95% CI 4.06–4.82).63 In a subgroup analysis for studies with BMI-matched populations, a similar risk was found (OR 4.00, 95% CI 1.97–8.10). The risk of developing hypertension and dyslipidaemia was also moderately increased compared to women without PCOS.
Forest plots of studies investigating a history of recurrent miscarriage in relation to the risk of fatal and non-fatal cardiovascular disease: (a) ischaemic heart disease; (b) stroke.
Considerations
Women with PCOS have a moderately increased overall CVD event risk. In addition, a higher risk of developing type 2 diabetes, hypertension and dyslipidaemia was found in women with PCOS.
Obesity is an important confounder in women with PCOS. Not all included studies adjusted for BMI. Therefore, it is uncertain whether obesity influences the development of CVD (risk factors) in women with PCOS. Non-obese women with PCOS have probably a lower risk of developing CVD than obese women with PCOS.
After adjustment for classic risk factors, the additional risk of CVD in women with PCOS might be limited. Therefore it is not indicated to perform cardiovascular screening in women with PCOS without ‘classic’ risk factors such as type 2 diabetes. Since the introduction of ovulation induction and IVF, most of the women with PCOS will conceive. The Dutch guideline on diabetes mellitus in pregnancy recommends that women with PCOS should be screened for gestational diabetes during pregnancy. Of pregnant women with PCOS, 22% develops gestational diabetes.65 In the Dutch guideline on type 2 diabetes it is recommended to screen women with gestational diabetes yearly for glucose intolerance, because these women are at high risk to develop type 2 diabetes,66 it is expected that the majority of women with PCOS who are predisposed to develop type 2 diabetes will be identified in pregnancy by the development and diagnosis of gestational diabetes.
Recommendations for cardiovascular risk management in women with a history of PCOS
The RR of developing CVD is lower than 2 in women with PCOS (level B evidence). Therefore, the guideline development group recommends no specific follow-up for these women. Nevertheless, the development group recommends that women with PCOS should be encouraged to optimise modifiable cardiovascular risk factors to reduce the risk of future CVD.
Premature ovarian insufficiency
Background
POI is characterised by amenorrhea, infertility and extended oestrogen deprivation in women aged less than 40 years, occurring in approximately 1% of the female population.7 In the majority of cases the cause underlying POI remains unknown.7 POI is associated with long-term female health implications that may include an increased incidence of CVD.67 It has been proposed that extended low oestrogen status at younger age leads to an increased risk of CVD events. However, the magnitude of CVD event risk associated with POI is currently unclear. This is because POI is a relatively rare condition and as a consequence most individual studies are hampered by small sample sizes. Also, women with POI are diagnosed at young age and consequently it takes longer to develop CVD events. In this section we determine the relation between spontaneous POI and CVD risk.
Summary of the literature
Recently, the results of the meta-analysis on the relation between CVD and POI were published in a more comprehensive review of the same authors as this guideline.68
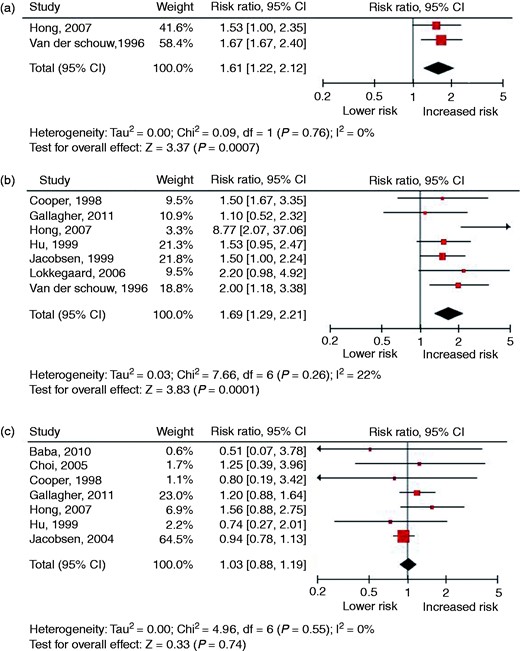
Ten cohort studies were identified. The sample sizes of the individual studies ranged from 2562 to 98,180 women and follow-up ranged from 4 to 37 years. Figure 8 presents the forest plots of studies investigating POI in relation to the risk of fatal and non-fatal CVD and Supplementary Table 8 presents the results of the meta-analysis from studies that have examined the relation between POI and the risk of CVD (risk factors). An increased risk of developing or dying from any CVD event (RR 1.61, 95% CI 1.22–2.12) and IHD (RR 1.69, 95% CI 1.29–2.21) was found. No relation was found for stroke (RR 1.03, 95% CI 0.88–1.19). Only one study assessed the relation between POI and the risk of having or developing hypertension and type 2 diabetes.
Forest plots of studies investigating polycystic ovary syndrome in relation to the risk of fatal and non-fatal cardiovascular disease: (a) overall cardiovascular disease; (b) ischaemic heart disease; (c) stroke.
Forest plots of studies investigating premature ovarian insufficiency in relation to the risk of fatal and non-fatal cardiovascular disease: (a) overall cardiovascular disease; (b) ischaemic heart disease; (c) stroke.
Considerations
Studies consistently showed that POI is associated with a significantly but modest increased risk of developing any CVD events and IHD. No increased risk of stroke was found in women with POI. As women in Europe develop stroke at a mean age of 75–80 years and follow-up of women with POI was limited, the lack of an association of POI with stroke in our meta-analysis might be explained by insufficient follow-up time. Most studies included the traditional CVD risk factors hypertension and type 2 diabetes as confounders in their analysis. Therefore, it can be concluded that POI is an independent although modest risk factor for IHD and overall CVD.
Until recently the widely accepted hypothesis was that the increased risk of developing CVD was caused by the withdrawal of oestrogen, which acts as a protective female hormone. Yet, an upcoming hypothesis is that early menopause is rather a consequence than a causal pathway for the development of CVD).69 More studies are needed to confirm this hypothesis.
Another important issue is hormone replacement therapy (HRT) use in women with POI as a possible important confounder of the association with CVD. Randomised clinical trials of HRT in postmenopausal women did not protect against CVD.70 Two of the included studies in our meta-analysis performed separate analyses to assess the effect of HRT on IHD risk.71,72 Jacobsen et al. found no difference in the risk of IHD in women with and without HRT (HR 1.5, 95% CI 1.0–2.3 vs. HR 1.7, 95% CI 1.0–2.8, respectively).72 Lokkegaard et al. found no effect of HRT in their natural early menopause group, although a protective effect of HRT was found in the surgical early menopause group.71 This suggests that the impact of HRT as a confounder on the observed relation in our meta-analysis may be limited.
Recommendations for cardiovascular risk management in women with a history of POI
The RR of developing CVD is lower than 2 in women with POI (level A2 evidence). Therefore, the guideline development group recommends no specific follow-up for these women. Nevertheless, the development group recommends that women with POI should be encouraged to optimise modifiable cardiovascular risk factors to reduce the risk of future CVD.
General discussion
The Dutch guideline on cardiovascular risk management after reproductive and pregnancy-related disorders is the first dedicated national guideline on this topic, addressing all pregnancy and reproductive disorders with suggested relations with CVD risk. Up to now few recommendations have been published addressing postpartum CVD prevention after reproductive and pregnancy-related disorders. These recommendations, most of them focusing on women with preeclampsia, are part of guidelines that were not developed for this particular group and/or are not based on systematic review of the available evidence and consensus between the engaged multidisciplinary healthcare professionals. A short summary of the different recommendations is given in Supplementary Table 9.
The guideline development group based its recommendations on the number and quality of the studies and the presence or absence of a RR > 2 of developing CVD events and/or risk factors from the meta-analysis. As a result, the development group only recommends follow-up and CVD risk factor screening after preeclampsia. It should be noted that up to now there are no intervention studies to support either this recommendation for women with preeclampsia or the lifestyle modifications the guideline recommends for women with any of the other reproductive and pregnancy-related disorders addressed in the guideline. However, excessive weight gain in pregnancy is associated with an increased risk of gestational diabetes and HDP and depends on the woman’s pre-pregnancy BMI.73 Also, years after delivery, women with preeclampsia have a higher BMI, as well as other cardiovascular risk factors such as unfavourable lipid profile and hyperglycaemia, compared to women with an uncomplicated pregnancy.74,75 These women will probably benefit most from lifestyle intervention strategies, including regular exercise, a healthy diet, achieving a desirable body weight and discontinuing smoking.28 Moreover, post-partum screening on cardiovascular risk factors and subsequent treatment in women with a history of GH or preeclampsia appear to be cost-effective with an increase in life expectancy.76
The prevention of pregnancy-related disorders is also an interesting approach. An increase of physical activity in and before pregnancy can reduce blood pressure and improve blood lipids profile leading to a reduction in preeclampsia incidence.77,78 Moreover, women who limit energy intake and are physically active during pregnancy are more likely to continue this lifestyle.79 Whether low-dosage aspirin started early in pregnancy reduces the risk of preeclampsia is still uncertain because meta-analyses on this topic showed conflicting results.80,81 The discrepancy between these meta-analyses could be explained by the dosage of aspirin administered, in which small RCTs with higher dosages of aspirin found a reduction in the risk of severe preeclampsia.81 The role of folic acid in the prevention of preeclampsia is uncertain and should be studied further.82
For this guideline the scientific literature was searched till the end of 2012. Therefore relevant articles published after this period were not included. These studies will be included in future updates of this guideline.
To establish uniformity and acceptance in The Netherlands, we decided to comply with the current Dutch guideline for cardiovascular risk management instead of the different international guidelines. Nevertheless, our recommendations can be implemented in other countries as well and women could be treated according to local cardiovascular risk management protocols.
Author contribution
KH, MB, CG, FD, NH, AH, JL, AM, JR, BV and AF contributed to the conception or design of the work. KH, MB, CG, FD, NH, AH, JL, AM, JR, BV and AF contributed to the acquisition, analysis, or interpretation of data for the work. KH, MB and AF drafted the manuscript. All authors critically revised the manuscript and gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Acknowledgements
This work was presented at the European Congress of the International Society for the Study of Hypertension in Pregnancy (ISSHP), 24–26 September 2015, Budapest, Hungary.
The authors gratefully acknowledge Miriam Cohen, general practitioner from the Center for Primary Care, Camper Practice, Amsterdam, for her contribution to this guideline. The authors would also like to acknowledge the advisory role of Teus van Barneveld at the Department of Quality in Healthcare of the Dutch Association of Medical Specialists in the development of this guideline and Monique Wessels for the help with the literature search.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The department of Obstetrics and Gynecology receives unrestricted educational grants from Ferring Pharmaceuticals. A Hoek received a grant from ZonMW (i.e. national Dutch scientific funding) for a RCT not related to this publication and as a member of the WOMB research consortium she received a grant from the Dutch Heart Foundation. She received a speaker’s fee from MSD for an educational oral.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This guideline was funded by a grant of the quality fund (SKMS) from the Dutch Society of Medical Specialists.




Comments