-
PDF
- Split View
-
Views
-
Cite
Cite
Carlo Torti, Franco Maggiolo, Andrea Patroni, Fredy Suter, Nicoletta Ladisa, Giuseppe Paraninfo, Piera Pierotti, Anna Maria Orani, Lorenzo Minoli, Claudio Arici, Laura Sighinolfi, Carmine Tinelli, Giampiero Carosi, for the MASTER Cohort, Exploratory analysis for the evaluation of lopinavir/ritonavir-versus efavirenz-based HAART regimens in antiretroviral-naive HIV-positive patients: results from the Italian MASTER Cohort, Journal of Antimicrobial Chemotherapy, Volume 56, Issue 1, July 2005, Pages 190–195, https://doi.org/10.1093/jac/dki172
Close - Share Icon Share
Abstract
Objective: This retrospective longitudinal cohort study compared the virological and immunological responses to highly active antiretroviral therapy containing either efavirenz or lopinavir/ritonavir in previously antiretroviral-naive HIV-infected patients.
Patients and methods: A total of 472 patients were selected (348 efavirenz and 124 lopinavir/ritonavir). The primary endpoint of this study was virological success (HIV RNA <50 copies/mL). The immunological response was assessed on the basis of either CD4+ T cell count variations (absolute and percentage) with respect to baseline values or categorical endpoints (defined as either a CD4+ T cell increase of ≥1;50 cells/mm3 at week 24 or of ≥1;75 cells/mm3 at week 48).
Results: At intention-to-treat (ITT) analysis, the adjusted odds ratio of virological success for patients who started lopinavir/ritonavir, compared with those who started efavirenz, was 0.54 (95% CI: 0.33–0.89, P = 0.016) at week 24 and 0.40 (95% CI: 0.33–0.89, P = 0.002) at week 48. However, patients receiving lopinavir/ritonavir had a more pronounced CD4+ T cell recovery, demonstrating both a mean absolute and percentage increase up to week 48 (MANOVA P < 0.0001).
Conclusions: Although comparisons of drug efficacy in non-randomized studies should be viewed with caution, from a virological point of view efavirenz-containing regimens performed as well (on-treatment analysis) or better (ITT analysis) than those containing lopinavir/ritonavir. In contrast, immunological outcome appeared to favour lopinavir/ritonavir.
Introduction
Multiple antiretroviral drugs belonging to four classes [nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTIs); non-nucleoside reverse transcriptase inhibitors (NNRTIs); protease inhibitors (PIs); and, recently, a fusion inhibitor] are currently available for antiretroviral therapy. Either NNRTI-based or PI-based highly active antiretroviral therapy (HAART) regimens are strongly recommended on the basis of existing efficacy data.1,2 PIs are preferably employed in combination with low-dose ritonavir (a so-called booster) to enhance plasma drug concentration and, therefore, antiretroviral activity.
On the one hand, a prospective randomized double-blind trial has demonstrated that lopinavir/ritonavir is virologically superior to nelfinavir in patients previously naive to antiretroviral therapy.3 On the other hand, the ACTG 384 study demonstrated the superiority of efavirenz in comparison with nelfinavir.4 On the basis of this evidence, either efavirenz- or lopinavir/ritonavir-based regimens are currently recommended by antiretroviral therapy guidelines as first choice HAART.1,2 However, there are few data directly comparing NNRTI-based regimens with boosted PI regimens.
In the absence of prospective randomized trial results, we decided to perform an exploratory analysis based on prospectively collected multicentre cohort data, to compare the effectiveness of efavirenz- versus lopinavir/ritonavir-containing HAART in patients previously naive to any antiretroviral treatment.
Patients and methods
Primary objective of the study
The primary objective of the study was to compare virological and immunological outcomes after first line HAART regimens (either including efavirenz or lopinavir/ritonavir) in HIV-positive patients naive to antiretrovirals.
Study design
This was a retrospective longitudinal analysis in patients enrolled in a multicentre cohort study.
Patients
Patient data were extracted from electronic databases (Health & Notes version 3.5, Healthware s.p.a, Naples, Italy) in use in 11 Italian clinical centres participating in the MASTER cohort.5 Patients who were previously antiretroviral naive, starting their first HAART regimen, which contained either efavirenz or lopinavir/ritonavir since contemporary availability of both drugs (January 1998), were selected at the coordinating centre, at which peripheral databases were merged. Only patients who were followed-up for at least 6 months were selected. A list of selected patients was then returned to each centre in order to fill in (available) incomplete data, and recheck data quality. Collected data were as follows: demographics, clinical stage of HIV disease (as by CDC 1993 classification, modified), risk factor for HIV acquisition, time of starting therapy, type of initial treatment and subsequent modifications, CD4+ T cell counts and HIV plasma viral load at baseline and during follow-up (normally performed after the first month of HAART and every 3 months thereafter, as recommended by antiretroviral therapy guidelines1,2). Follow-up was censored at week 48, at permanent therapy discontinuation, or ‘patient lost to follow-up’, whichever came first.
Study endpoints
The primary virological endpoint of the study was virological success (i.e. plasma viral load <50 copies/mL) either at weeks 24 or 48. A secondary virological endpoint was the proportion of patients whose virological response was sustained from week 24 up to week 48.
The immunological response was primarily assessed on the basis of CD4+ T cell count variations (absolute and percentage) with respect to baseline values considered as continuous measures. The immunological response was also assessed by a categorical endpoint—‘low CD4+ response’ defined either as a CD4+ T cell increase of <50 cells/mm3 at week 24 or of <75 cells/mm3 at week 48, as a reference.6
Statistics
Rates of undetectable plasma viral load. Owing to the observational nature of this study, in the primary analysis patients were considered as belonging to the initial study treatment regimen during the entire follow-up, even if initial efavirenz or lopinavir/ritonavir were discontinued [intention-to-treat (ITT)]. Therapy switches or interruptions were not considered as failures in this analysis, however. A further (secondary) analysis was conducted censoring data when efavirenz or lopinavir/ritonavir were discontinued (on-treatment), while changes occurring in the N(t)RTI backbone were always ignored. Patients whose data were missing at any individual time-point were retained in the analysis provided that further follow-up was available; however, these patients were excluded from the denominator in the efficacy analyses at points coincident with missing data.
Patient sample size allowed the detection of a 15% difference in the probability of reaching undetectable plasma viral load, with a power of 84 and 79% at the beginning and at the end of follow-up, respectively.
Analyses were repeated in patients whose HIV infection was more advanced (i.e. CD4+ T cell count ≤200/mm3 or HIV plasma viral load >100000 copies/mL), and in those whose viro-immunological status was less compromised, taken separately.
Logistic regression analysis. The main logistic regression analysis of outcome predictors was conducted using three separate models based on the primary (probability of achieving undetectable viral load either at weeks 24 or 48) and secondary (sustained response) virological endpoints.
The following independent variables were used in all analyses: gender, age, risk factor for HIV acquisition, clinical stage of HIV disease, baseline CD4+ T cell count, baseline HIV plasma viral load, nadir CD4+ T cell count from baseline up to termination of the follow-up, treatment group (efavirenz versus lopinavir/ritonavir), type of N(t)RTI in the associated backbone, clinical centre and time of treatment initiation.
Analysis of immunological outcome. Time-trend adjusted analysis (MANOVA) was used to compare CD4+ T cell count variations (absolute and percentage) with respect to baseline values considered as continuous measures. Normal distribution of the response variable was assessed and then a generalized estimating equation (GEE) was used to fit the linear regression models in order to investigate the relationship between CD4+ percentage change over the entire 48 week follow-up and possible explanatory variables both at univariate and multivariate analysis. The above factors were used in the GEE model. Further logistic regression modelling was performed to explore the immunological response considered as a categorical variable, in which sustained undetectable (yes/no) plasma viral load was considered as a further variable.
Additional statistical notes. Only variables reaching a P value ≤0.20 in univariate models were entered into multivariate models. P values <0.05 were considered to be statistically significant in these models. All statistical analyses were performed with STATA (StataCorp 2000. Stata Statistical Software: release 7.0; College Station, TX, USA) and STATISTICA for Windows (StatSoft, Inc. 2000; Tulsa, OK, USA).
Results
Patients
A total of 472 patients were selected (348 efavirenz and 124 lopinavir/ritonavir). As illustrated in Table 1, patient age, gender, risk factor for HIV acquisition and HIV plasma viral load did not differ significantly between the two groups. In contrast, patients who were prescribed lopinavir/ritonavir-containing regimens had a more advanced HIV disease stage, as demonstrated by the higher proportion of CDC class C (32.3 versus 23%, P = 0.04), the higher percentage of patients whose CD4+ T cell count was <200 cells/mm3 (51 versus 33%, P = 0.0006), and the lower mean CD4+ T cell count (176 cells/mm3 versus 215 cells/mm3, P = 0.03).
Baseline characteristics of the study patients
| Characteristics . | EFV group (n = 348) . | LPV/r group (n = 124) . | P . | |||
|---|---|---|---|---|---|---|
| Mean age [years] (SD) | 38.7 (8.6) | 39.3 (9.5) | 0.47 | |||
| Male gender (%) | 78.2 | 75.8 | 0.59 | |||
| IVDU risk factor (%) | 27.3 | 25.0 | 0.60 | |||
| Mean HIV-RNA [log10 copies/mL] (SD) | 4.8 (0.7) | 4.9 (0.8) | 0.14 | |||
| HIV RNA < 100 000 copies/mL (%) | 54.0 | 49.2 | 0.35 | |||
| Mean CD4+ cell count (cells/mm3) ± SD | 214.6 ± 152.3 | 176.4 ± 191.0 | 0.03 | |||
| CD4+ cell count <200 cells/mm3 (%) | 49.1 | 66.9 | 0.0006 | |||
| Class C according to CDC '93 (%) | 22.9 | 32.3 | 0.04 | |||
| N(t)RTI in the backbone (%) | ||||||
| ZDV | 43.4 | 78.2 | <0.0001 | |||
| d4T | 19.8 | 13.7 | 0.13 | |||
| 3TC | 87.1 | 92.7 | 0.09 | |||
| ddI | 31.3 | 7.3 | <0.0001 | |||
| ABC | 8.9 | 0.8 | 0.002 | |||
| TDF | 13.2 | 8.1 | 0.13 | |||
| Characteristics . | EFV group (n = 348) . | LPV/r group (n = 124) . | P . | |||
|---|---|---|---|---|---|---|
| Mean age [years] (SD) | 38.7 (8.6) | 39.3 (9.5) | 0.47 | |||
| Male gender (%) | 78.2 | 75.8 | 0.59 | |||
| IVDU risk factor (%) | 27.3 | 25.0 | 0.60 | |||
| Mean HIV-RNA [log10 copies/mL] (SD) | 4.8 (0.7) | 4.9 (0.8) | 0.14 | |||
| HIV RNA < 100 000 copies/mL (%) | 54.0 | 49.2 | 0.35 | |||
| Mean CD4+ cell count (cells/mm3) ± SD | 214.6 ± 152.3 | 176.4 ± 191.0 | 0.03 | |||
| CD4+ cell count <200 cells/mm3 (%) | 49.1 | 66.9 | 0.0006 | |||
| Class C according to CDC '93 (%) | 22.9 | 32.3 | 0.04 | |||
| N(t)RTI in the backbone (%) | ||||||
| ZDV | 43.4 | 78.2 | <0.0001 | |||
| d4T | 19.8 | 13.7 | 0.13 | |||
| 3TC | 87.1 | 92.7 | 0.09 | |||
| ddI | 31.3 | 7.3 | <0.0001 | |||
| ABC | 8.9 | 0.8 | 0.002 | |||
| TDF | 13.2 | 8.1 | 0.13 | |||
EFV, efavirenz; LPV/r, lopinavir/ritonavir; IVDU, intravenous drug use; ZDV, zidovudine; d4T, stavudine; 3TC, lamivudine; ddI, didanosine; ABC, abacavir; TDF, tenofovir; N(t)RTI, nucleoside or nucleotide reverse transcriptase inhibitors.
Baseline characteristics of the study patients
| Characteristics . | EFV group (n = 348) . | LPV/r group (n = 124) . | P . | |||
|---|---|---|---|---|---|---|
| Mean age [years] (SD) | 38.7 (8.6) | 39.3 (9.5) | 0.47 | |||
| Male gender (%) | 78.2 | 75.8 | 0.59 | |||
| IVDU risk factor (%) | 27.3 | 25.0 | 0.60 | |||
| Mean HIV-RNA [log10 copies/mL] (SD) | 4.8 (0.7) | 4.9 (0.8) | 0.14 | |||
| HIV RNA < 100 000 copies/mL (%) | 54.0 | 49.2 | 0.35 | |||
| Mean CD4+ cell count (cells/mm3) ± SD | 214.6 ± 152.3 | 176.4 ± 191.0 | 0.03 | |||
| CD4+ cell count <200 cells/mm3 (%) | 49.1 | 66.9 | 0.0006 | |||
| Class C according to CDC '93 (%) | 22.9 | 32.3 | 0.04 | |||
| N(t)RTI in the backbone (%) | ||||||
| ZDV | 43.4 | 78.2 | <0.0001 | |||
| d4T | 19.8 | 13.7 | 0.13 | |||
| 3TC | 87.1 | 92.7 | 0.09 | |||
| ddI | 31.3 | 7.3 | <0.0001 | |||
| ABC | 8.9 | 0.8 | 0.002 | |||
| TDF | 13.2 | 8.1 | 0.13 | |||
| Characteristics . | EFV group (n = 348) . | LPV/r group (n = 124) . | P . | |||
|---|---|---|---|---|---|---|
| Mean age [years] (SD) | 38.7 (8.6) | 39.3 (9.5) | 0.47 | |||
| Male gender (%) | 78.2 | 75.8 | 0.59 | |||
| IVDU risk factor (%) | 27.3 | 25.0 | 0.60 | |||
| Mean HIV-RNA [log10 copies/mL] (SD) | 4.8 (0.7) | 4.9 (0.8) | 0.14 | |||
| HIV RNA < 100 000 copies/mL (%) | 54.0 | 49.2 | 0.35 | |||
| Mean CD4+ cell count (cells/mm3) ± SD | 214.6 ± 152.3 | 176.4 ± 191.0 | 0.03 | |||
| CD4+ cell count <200 cells/mm3 (%) | 49.1 | 66.9 | 0.0006 | |||
| Class C according to CDC '93 (%) | 22.9 | 32.3 | 0.04 | |||
| N(t)RTI in the backbone (%) | ||||||
| ZDV | 43.4 | 78.2 | <0.0001 | |||
| d4T | 19.8 | 13.7 | 0.13 | |||
| 3TC | 87.1 | 92.7 | 0.09 | |||
| ddI | 31.3 | 7.3 | <0.0001 | |||
| ABC | 8.9 | 0.8 | 0.002 | |||
| TDF | 13.2 | 8.1 | 0.13 | |||
EFV, efavirenz; LPV/r, lopinavir/ritonavir; IVDU, intravenous drug use; ZDV, zidovudine; d4T, stavudine; 3TC, lamivudine; ddI, didanosine; ABC, abacavir; TDF, tenofovir; N(t)RTI, nucleoside or nucleotide reverse transcriptase inhibitors.
Furthermore, the type of prescribed N(t)RTIs used as backbone in HAART regimens differed. Patients in the lopinavir/ritonavir group received zidovudine (P < 0.0001) and lamivudine (P = 0.09) more frequently, while patients in the efavirenz group were more frequently prescribed didanosine (P < 0.001) and abacavir (P = 0.02). There were no statistically significant differences in mean length of follow-up between the efavirenz [348.4 days (SD: 30.5)] and the lopinavir/ritonavir [342.2 days (SD: 38.2)] groups (P = 0.07).
Virological outcome and predictors
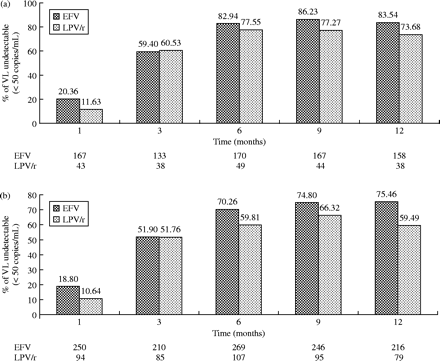
Bearing in mind the percentage of patients with an undetectable HIV plasma viral load at any single time-point of the study, the on-treatment analysis showed no statistically significant differences in the entire follow-up period (Figure 1a). In contrast, using ITT analysis (Figure 1b), patients in the efavirenz group tended to have higher percentages of undetectable viral load than those in the lopinavir/ritonavir group (19 versus 11% at week 4, P = 0.07; 70 versus 60% at week 24, P = 0.05; 75 versus 59%, P = 0.007 at week 48).
Virological response over time. Proportion of patients with HIV-RNA <50 copies/mL at different time points. (a) On-treatment analysis and (b) ITT analysis. EFV, efavirenz; LPV/r, lopinavir/ritonavir. The number of patients whose data were available at each time-point of follow-up are indicated under the horizontal axis.
When patients were analysed according to their viro-immunological status at baseline, the benefit found at ITT analysis could be explained by a statistically significant higher proportion of undetectable HIV plasma viral load in patients with a baseline CD4+ T cell count >200 cells/mm3 who were prescribed efavirenz, with respect to those who were prescribed lopinavir/ritonavir. No statistically significant differences were found in rates of undetectable viral load between patients who had CD4+ T cell counts ≤200 cells/mm3 at baseline and who were treated with either efavirenz or lopinavir/ritonavir. Similarly, efavirenz-containing regimens appeared to induce a higher benefit in patients who had a baseline HIV viral load <100 000 copies/mL at ITT analysis, with respect to those in the lopinavir/ritonavir group (31 versus 15.2% at week 4, P = 0.04; 73.5 versus 51.3% at week 48, P = 0.008), while no difference was observed among patients with higher (≥100 000 copies/mL) viral loads.
Table 2 shows variables that were independently associated with virological outcome. According to the ITT approach, the probability of obtaining an undetectable viral load at week 24 was significantly lower for patients prescribed lopinavir/ritonavir (OR: 0.54, 95% CI: 0.33–0.89, P = 0.016) or didanosine (OR: 0.52, 95% CI: 0.30–0.89, P = 0.018) and for those with a baseline HIV plasma viral load ≥100 000 copies/mL (OR: 0.57, 95% CI: 0.36–0.90, P = 0.015), while age was associated with better outcome per unit-year increase (OR: 1.04, 95% CI: 1.01–1.06, P = 0.01). At week 48, lopinavir/ritonavir use still acted as an independent risk factor for the lower probability of achieving an undetectable viral load (OR: 0.37, 95% CI: 0.20–0.66, P = 0.001). In the on-treatment analysis, the use of efavirenz or lopinavir/ritonavir was not associated with the probability of obtaining an undetectable viral load either at weeks 24 or 48. Finally, only the nadir CD4+ T cell count (per each lower CD4+ T cell) was independently correlated with the risk of not achieving a sustained virological response at weeks 24 and 48 (OR: 0.998, 95% CI: 0.997–0.999, P = 0.04).
Predictive values of different baseline characteristics on the virological outcome (HIV RNA <50 copies/mL either at weeks 24 or 48 according to ITT analysis)a
| . | Week 24 . | . | . | . | Week 48 . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted (n: 376) . | . | Adjusted (max n: 376) . | . | Unadjusted (n: 295) . | . | Adjusted (max n: 295) . | . | ||||||||
| Factor . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||||||||
| Group (LPV/r versus EFV) | 0.63 (0.39–1.00) | 0.05 | 0.54 (0.33–0.89) | 0.016 | 0.48 (0.28–0.82) | 0.008 | 0.37 (0.20–0.66) | 0.001 | ||||||||
| Age (per unit-year increase) | 1.03 (1.00–1.06) | 0.03 | 1.04 (1.01–1.06) | 0.01 | 1.03 (1.00–1.06) | 0.09 | – | – | ||||||||
| CD4+ T cell count (≥200 versus <200 cells/mm3) | – | – | – | – | 0.53 (0.31–0.87) | 0.01 | 0.46 (0.27–0.78) | 0.004 | ||||||||
| HIV plasma viral load (≥100 000 versus <100 000 copies/mL) | 0.62 (0.40–0.96) | 0.03 | 0.57 (0.36–0.90) | 0.015 | – | – | – | – | ||||||||
| N(t)RTI in the backbone (yes versus no) | ||||||||||||||||
| ddI | 0.64 (0.38–1.06) | 0.08 | 0.52 (0.30–0.89) | 0.018 | – | – | – | – | ||||||||
| ABC | – | – | – | – | 0.43 (0.16–1.16) | 0.09 | 0.34 (0.12–0.96) | 0.04 | ||||||||
| . | Week 24 . | . | . | . | Week 48 . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted (n: 376) . | . | Adjusted (max n: 376) . | . | Unadjusted (n: 295) . | . | Adjusted (max n: 295) . | . | ||||||||
| Factor . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||||||||
| Group (LPV/r versus EFV) | 0.63 (0.39–1.00) | 0.05 | 0.54 (0.33–0.89) | 0.016 | 0.48 (0.28–0.82) | 0.008 | 0.37 (0.20–0.66) | 0.001 | ||||||||
| Age (per unit-year increase) | 1.03 (1.00–1.06) | 0.03 | 1.04 (1.01–1.06) | 0.01 | 1.03 (1.00–1.06) | 0.09 | – | – | ||||||||
| CD4+ T cell count (≥200 versus <200 cells/mm3) | – | – | – | – | 0.53 (0.31–0.87) | 0.01 | 0.46 (0.27–0.78) | 0.004 | ||||||||
| HIV plasma viral load (≥100 000 versus <100 000 copies/mL) | 0.62 (0.40–0.96) | 0.03 | 0.57 (0.36–0.90) | 0.015 | – | – | – | – | ||||||||
| N(t)RTI in the backbone (yes versus no) | ||||||||||||||||
| ddI | 0.64 (0.38–1.06) | 0.08 | 0.52 (0.30–0.89) | 0.018 | – | – | – | – | ||||||||
| ABC | – | – | – | – | 0.43 (0.16–1.16) | 0.09 | 0.34 (0.12–0.96) | 0.04 | ||||||||
Only factors that were found to be significantly associated with the virological outcome in the multivariate model are shown here. Hosmer–Lemeshow χ2 were, respectively, 4.59 (probability >χ2: 0.7999) and 0.13 (probability >χ2: 0.9383) for the multivariate models performed at months 6 and 12.
Predictive values of different baseline characteristics on the virological outcome (HIV RNA <50 copies/mL either at weeks 24 or 48 according to ITT analysis)a
| . | Week 24 . | . | . | . | Week 48 . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted (n: 376) . | . | Adjusted (max n: 376) . | . | Unadjusted (n: 295) . | . | Adjusted (max n: 295) . | . | ||||||||
| Factor . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||||||||
| Group (LPV/r versus EFV) | 0.63 (0.39–1.00) | 0.05 | 0.54 (0.33–0.89) | 0.016 | 0.48 (0.28–0.82) | 0.008 | 0.37 (0.20–0.66) | 0.001 | ||||||||
| Age (per unit-year increase) | 1.03 (1.00–1.06) | 0.03 | 1.04 (1.01–1.06) | 0.01 | 1.03 (1.00–1.06) | 0.09 | – | – | ||||||||
| CD4+ T cell count (≥200 versus <200 cells/mm3) | – | – | – | – | 0.53 (0.31–0.87) | 0.01 | 0.46 (0.27–0.78) | 0.004 | ||||||||
| HIV plasma viral load (≥100 000 versus <100 000 copies/mL) | 0.62 (0.40–0.96) | 0.03 | 0.57 (0.36–0.90) | 0.015 | – | – | – | – | ||||||||
| N(t)RTI in the backbone (yes versus no) | ||||||||||||||||
| ddI | 0.64 (0.38–1.06) | 0.08 | 0.52 (0.30–0.89) | 0.018 | – | – | – | – | ||||||||
| ABC | – | – | – | – | 0.43 (0.16–1.16) | 0.09 | 0.34 (0.12–0.96) | 0.04 | ||||||||
| . | Week 24 . | . | . | . | Week 48 . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted (n: 376) . | . | Adjusted (max n: 376) . | . | Unadjusted (n: 295) . | . | Adjusted (max n: 295) . | . | ||||||||
| Factor . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||||||||
| Group (LPV/r versus EFV) | 0.63 (0.39–1.00) | 0.05 | 0.54 (0.33–0.89) | 0.016 | 0.48 (0.28–0.82) | 0.008 | 0.37 (0.20–0.66) | 0.001 | ||||||||
| Age (per unit-year increase) | 1.03 (1.00–1.06) | 0.03 | 1.04 (1.01–1.06) | 0.01 | 1.03 (1.00–1.06) | 0.09 | – | – | ||||||||
| CD4+ T cell count (≥200 versus <200 cells/mm3) | – | – | – | – | 0.53 (0.31–0.87) | 0.01 | 0.46 (0.27–0.78) | 0.004 | ||||||||
| HIV plasma viral load (≥100 000 versus <100 000 copies/mL) | 0.62 (0.40–0.96) | 0.03 | 0.57 (0.36–0.90) | 0.015 | – | – | – | – | ||||||||
| N(t)RTI in the backbone (yes versus no) | ||||||||||||||||
| ddI | 0.64 (0.38–1.06) | 0.08 | 0.52 (0.30–0.89) | 0.018 | – | – | – | – | ||||||||
| ABC | – | – | – | – | 0.43 (0.16–1.16) | 0.09 | 0.34 (0.12–0.96) | 0.04 | ||||||||
Only factors that were found to be significantly associated with the virological outcome in the multivariate model are shown here. Hosmer–Lemeshow χ2 were, respectively, 4.59 (probability >χ2: 0.7999) and 0.13 (probability >χ2: 0.9383) for the multivariate models performed at months 6 and 12.
Immunological outcome and predictors
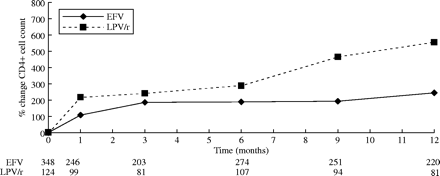
Regarding immunological response, CD4+ T cell recovery was more pronounced in patients in whom lopinavir/ritonavir was prescribed at the ITT analysis, either in terms of a mean absolute or percentage increase up to week 48 (P < 0.0001, MANOVA test; Figure 2). On-treatment analysis results did not differ significantly. No statistically significant differences were found between groups as far as the categorical immunological endpoints were considered, either at weeks 24 or 48. For instance, at the on-treatment analysis conducted at week 24, 165/216 (76.4%) and 55/76 (72.4%) patients gained at least 50 cells/mm3 in the efavirenz and lopinavir/ritonavir groups, respectively (P = 0.48). At week 48, the proportion of patients who gained at least 75 CD4+ T cells/mm3 were, respectively, 129/162 (79.6%) and 29/37 (78.4%) (P = 0.86).
CD4+ T cell variation (percent age change compared with the baseline value) over time according to treatment. EFV, efavirenz; LPV/r, lopinavir/ritonavir. The number of patients whose data were available at each time-point of follow-up are indicated under the horizontal axis.
A time-adjusted generalized estimation equation model demonstrated that factors independently associated with a higher percentage CD4+ T cell increase were as follows: use of lopinavir/ritonavir (coefficient 110.2; 95% CI: 17.6–202.7; P = 0.02), stavudine + lamivudine backbone (coefficient 122; 95% CI: 3.3–240.7; P = 0.044) and advanced clinical and viro-immunological conditions at baseline. By contrast, none of the factors that were assessed in the multivariate models were associated with a CD4+ T cell increase above the categorical endpoints, apart from sustained HIV viral load <50 copies/mL at weeks 24 and 48 for the probability of gaining at least 75 CD4+ T cells/mm3 over baseline at week 48 (OR: 2.92; 95% CI: 0.99–8.59, P = 0.051).
Treatment discontinuations and patients lost to follow-up
Overall, 99/472 (21%) patients discontinued efavirenz or lopinavir/ritonavir before week 48 (65/348 = 18.7% of patients who were prescribed efavirenz and 34/124 = 27.4% of those who were prescribed lopinavir/ritonavir; P = 0.047). Among reasons for discontinuation, the main cause was treatment-limiting toxicity, which occurred in 31/65 (48%) of patients prescribed efavirenz and in 14/34 (41.2%) of those prescribed lopinavir/ritonavir. The second most frequent cause was patient willingness to discontinue, occurring in 12/65 (18.5%) in the efavirenz and in 5/34 (14.7%) in the lopinavir/ritonavir group. Other causes of discontinuation in patients taking efavirenz or lopinavir/ritonavir were, respectively, as follows: treatment simplification strategy (1/65 = 1.6% and 7/34 = 20.6%), viro-immunological failure (5/65 = 7.7% and 1/34 = 2.9%), lost-to-follow-up (2/65 = 3.1% and 1/34 = 2.9%) and death (2/65 = 3.1% and 0/34 = 0%). Reasons for treatment discontinuation were not retrieved in 19/99 (19.2%) patients. Finally, patients lost to follow-up in the efavirenz and lopinavir/ritonavir groups were, respectively: 30 and 12 between weeks 24 and 36; and 82 and 29 between weeks 36 and 48.
Discussion
It is uncertain whether NNRTI or boosted PI-based regimens are preferable as initial treatment, owing to the paucity of available comparative data. In the FOCUS trial,7 an efavirenz-based regimen was compared with ritonavir-boosted soft-gel saquinavir, resulting in better virological control and less toxicity. Moreover, efavirenz performed better than a ritonavir-boosted amprenavir-based regimen.8 However, despite the fact that comparative data between lopinavir/ritonavir- and efavirenz-based regimens are lacking, both these regimens are currently recommended as first choice initial antiretroviral therapy.1,2 For this reason, we decided to conduct an exploratory analysis, aimed at comparing efavirenz-based with lopinavir/ritonavir-based regimens in clinical practice.
There are intrinsic limitations in such cohort analysis, essentially due to the fact that patients are not randomized to one regimen or another, and that patient management is not standardized. In the case of our study, these methodological drawbacks were limited thanks to the broad implementation of national guidelines that suggested that both efavirenz and lopinavir/ritonavir are recommended as first line therapies in guided management of HIV chronically infected patients.9 Furthermore, the basic analysis performed in this study was based on a complete follow-up irrespective of treatment changes that occurred in single patients, thus enabling an evaluation of treatment strategies rather than regimens.10 Finally, our approach limited interference due to switches of therapy, which is a clinician-driven endpoint, and the practice of toxicity management.11
Nevertheless, we cannot rule out the possibility that some differences between the two groups of patients could influence the final outcome. For instance, patients who were prescribed efavirenz received N(t)RTI—which are prescribed ‘once daily’—more frequently than those who were prescribed lopinavir/ritonavir, therefore allowing the construction of ‘once daily’ regimens. Such a treatment simplification strategy may have allowed patients to adhere to treatment more easily,12 therefore compensating (and masking) possible differences in intrinsic potency between these two kinds of regimens. Unfortunately, reliable data regarding treatment adherence were lacking in this study. On the other hand, the inclusion of didanosine in the regimen appeared to exert an unfavourable impact on the virological treatment response at the 24 week follow-up analysis. This evidence was surprising since didanosine was more frequently prescribed together with efavirenz whose activity was found to be at least equal to the lopinavir/ritonavir comparator arm. Therefore, the association between didanosine use and the lower probability of achieving virological suppression may be explained by the small number of patients who were prescribed didanosine and it may have occurred by chance. It is also necessary to underline that the apparent negative influence of didanosine was only detected at the 24 week follow-up analysis, and not at the later time-point, possibly indicating only slower activity of regimens containing this drug. However, a recent study has described a high rate of virological failure in patients on tenofovir/didanosine/efavirenz,13 but fewer than 1% of the study patients received this association in our cohort. Didanosine was prescribed with stavudine in around 4% of patients in both groups. This combination is currently contraindicated by antiretroviral therapy guidelines on the basis of a higher toxicity risk.2 Finally, didanosine is the only NRTI whose consumption is subordinated to diet requirements, thus rendering treatment regimens more complicated, and this may have decreased adherence, although this variable could not have been captured in this study.
ITT analysis showed that efavirenz-containing regimens performed significantly better than lopinavir/ritonavir from a virological point of view. Another study,14 showed that the effectiveness of NNRTI-based treatment was better than that of PI-based regimens; however, in that study most patients were on single PI regimens (with a small portion on lopinavir/ritonavir). Therefore, our results extend those reported in the current literature to a significant extent. Better effectiveness of efavirenz-containing regimens was particularly evident in patients whose immunological status was less advanced at baseline and was independent from the impact exerted by high HIV plasma viral load in our study.
According to on-treatment analysis, no significant differences between the two regimens were observed as far as the virological response was concerned. However, patients were not balanced at baseline since those belonging to the lopinavir/ritonavir group had more advanced HIV infection and the N(t)RTI backbone differed. Notwithstanding this, we do not feel that differences between patients at baseline could have affected the final outcome to a significant extent. In fact, analysis conducted in patients presenting lower CD4 T cell counts did not yield any statistically significant difference between those treated with lopinavir/ritonavir and those treated with efavirenz-containing regimens.
An interesting observation of this study was that CD4+ T cell increase, analysed as a continuous outcome measure, seemed to favour lopinavir/ritonavir-based regimens in the overall patient population, as previously reported.15,16 The superiority of lopinavir/ritonavir-containing regimens was confirmed at multivariate analysis; however, it was not confirmed when pre-defined cut-off levels were used to judge immunological response as reference.6
The present study has several limitations which have to be recognized. Although there were 472 patients in the study, the actual sample size at 48 weeks of follow-up was considerably lower, thus limiting statistical power. Moreover, the nadir CD4+ T cell count was independently correlated with the probability of achieving a sustained virological response; as patients who were prescribed lopinavir/ritonavir had a more advanced HIV disease, this may have influenced results.
In conclusion, this exploratory analysis suggests that the virological effectiveness of efavirenz-containing regimens is similar or better than those containing lopinavir/ritonavir, while immunological improvement seemed to favour lopinavir/ritonavir-containing regimens rather than those containing efavirenz. Based on these considerations, we conclude that both drugs might be considered as first line choices for the treatment of chronic HIV infection. In clinical practice, several considerations should be borne in mind when choosing a first line HAART regimen. These should include not only viro-immunological efficacy but also patient preference and lifestyle, concomitant pathologies and treatments, and the care giver's experience with either drug. A prospective randomized study (Si.S.Ther. – Simplified, Sequencing Therapy) is ongoing in our centres, aiming at individualizing treatment prescription in patients starting HAART.
The following persons are members of the Italian Master Cohort: Cristini G., Casari S, Castelnuovo F., Forleo M.A., Lapadula G., Moretti F., Nasta P., Paraninfo G., Patroni A., Quiros-Roldan E., Tirelli V., Torti C., Uccelli M.C., Carosi G. (Brescia); Arici C., Maggiolo F., Ripamonti D., Suter F. (Bergamo); Ladisa N., Pastore G. (Bari); Antinori A., Antonucci G. (INMI-Rome);Lo Caputo S., Mazzotta F. (Firenze); Maserati R., Novati S.,Minoli L. (Pavia), Sighinolfi L., Ghinelli F. (Ferrara); Mondello P., Carnevale G. (Cremona); Dionisio D., Vivarelli A. (Pistoia); Rizzardini G., Migliorino P. (Busto Arsizio); Labate L., Tinelli C. (Statistical Unit).
References
Yeni PG, Hammer SM, Hirsch MS et al. Treatment for adult HIV infection. 2004 recommendations of the International AIDS Society-USA Panel.
Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Updated 29 October
Walmsey S, Bernstein B, King M et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection.
Robbins GK, De Gruttola V, Shaker RW et al. Comparison of sequential three drug regimens as initial therapy for HIV infection.
Carosi G, Castelli F, Suter F et al. Antiviral potency of HAART regimens and clinical success are not strictly couplet in real life conditions: evidence from the MASTER-1 study.
Florence E, Lundgren J, Dreezen C et al. Factors associated with a reduced CD4 lymphocyte count responses to HAART despite full viral suppression in the EuroSIDA study.
Montaner JSG, Saag MS, Barylski C et al. FOCUS study: saquinavir QD regimen versus efavirenz QD regimen 48 week analysis in HIV infected patients. In: Programs and Abstracts of the Forty-second Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, 2002. Abstract H-167, p. 261. American Society for Microbiology, Washington, DC, USA.
Bartlett JA, Johnson J, Herrera G et al. Initial therapy with abacavir + lamivudine (ABC + 3TC) combined with efavirenz (NNRTI), amprenavir/ritonavir (PI) or stavudine (NRTI): ESS40001 (CLASS). In: Program and Abstracts of the XV International AIDS conference, Bangkok, Thailand, 2004. Abstract TuPeB4544. http://www.ejais.com
Commissione Nazionale per la Lotta Contro l'AIDS e le Altre Malattie Infettive Emergenti e Riemergenti. Aggiornamento sulle conoscenze in tema di terapia antiretrovirale. http://www.anlaids.it. (1 December 2004, date last accessed).
Kirk O, Pedersen C, Law M et al. Analysis of virological efficacy in trials of antiretroviral regimens: drawbacks of not including viral load measurements after premature discontinuation of therapy.
Kirk O, Lundgren JD. Clinical trial methodology and clinical cohorts: the importance of complete follow-up in trials evaluating the virologic efficacy of anti-HIV medicines.
Maggiolo F, Ravasio L, Ripamonti D et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors.
Podzamczer D, Ferrer E, Gatell JM et al. Early virologic failure with a combination of tenofovir, didanosine and efavirenz.
Klein MB, Willemot P, Murphy T et al. The impact of initial highly active antiretroviral therapy on future treatment sequences in HIV infection.
Barreiro P, Soriano V, Casas E et al. Different degree of immune recovery using antiretroviral regimens with protease inhibitors or non-nucleosides.
Author notes
1Institute for Infectious and Tropical Diseases, University of Brescia, Italy; 2Department of Infectious Diseases, Bergamo, Italy; 3Biostatistics Unit, IRCCS Policlinico S. Matteo, Pavia, Italy; 4Institute of Infectious Diseases, University of Bari, Bari, Italy; 5Department of Infectious Diseases, S.M. Annunziata Hospital, Florence, Italy; 6Department of Infectious Diseases, ‘A. Manzoni’ Hospital, Lecco, Italy; 7Institute of Infectious Diseases, University of Pavia, Pavia, Italy; 8Department of Infectious Diseases, Ferrara, Italy