-
PDF
- Split View
-
Views
-
Cite
Cite
Dick Menzies, What Does Tuberculin Reactivity after Bacille Calmette-Guérin Vaccination Tell Us?, Clinical Infectious Diseases, Volume 31, Issue Supplement_3, September 2000, Pages S71–S74, https://doi.org/10.1086/314075
Close - Share Icon Share
Abstract
The effect of bacille Calmette-Guérin (BCG) vaccination on tuberculin reactivity is briefly reviewed. BCG vaccination will almost invariably result in tuberculin conversion with a positive tuberculin skin test developing 4–8 weeks after vaccination. However, these tuberculin reactions will wane—rapidly in all individuals who receive the vaccine in the neonatal period and more slowly in those who are vaccinated at an older age such as during the primary-school years. Of BCG vaccine recipients whose initial tuberculin skin test is negative, 10%–25% will have a positive tuberculin skin test if they are retested within 1–4 weeks—the so-called “booster phenomenon.” There is no relationship between tuberculin reactivity after BCG vaccination and the protective efficacy of the vaccine against development of active tuberculosis. Therefore, the ideal BCG vaccine would produce a scar at the site of injection to identify individuals who have been vaccinated but would have no effect on tuberculin reactivity.
Tuberculin reactivity after BCG vaccination has been the most common measure of the effect of the BCG vaccine. There is extensive literature on the influence of the vaccine manufacturer's technique of administration, the study population, and the age of subjects at vaccination on tuberculin reactivity as well as literature on the effect of interval and techniques of testing, such as the two-step protocol to elicit the booster phenomenon. As a result, more is known about the effect on tuberculin reactivity than any other aspect of BCG vaccination. There is very limited information on the clinical efficacy of the BCG vaccine in terms of prevention of primary infection with Mycobacterium tuberculosis, and information on the efficacy of the BCG vaccine in the prevention of the development of disease with M. tuberculosis is contradictory and inconsistent. In the search for a new and more effective vaccine, information regarding the effect of vaccination on tuberculin reactivity should be reviewed carefully, because it may provide important insights into the immunogenic activity and protective efficacy of the BCG vaccine.
What Is the Effect of BCG Vaccination on Tuberculin Reactivity?
Studies involving many different vaccines, populations, and age groups have consistently demonstrated that >90% of BCG vaccine recipients will have tuberculin reactions ⩾10 mm in diameter develop within 8–12 weeks after vaccination. Eight weeks after vaccination with BCG strains manufactured by 1 of 10 different manufacturers, tuberculin reactions were not significantly different among 6-year-old Danish schoolchildren, with the exception of reactions to BCG strains from one manufacturer in East Germany [1].
The effect of vaccine dose was first examined in a study of 1513 Danish infants who were randomized to receive doses in serial twofold dilutions down to 1/32 of the standard adult dose (0.75 mg of Danish State Serum Institute BCG) [2]. There was a clear dose-response relationship between vaccine dose and the size of lesions at the site of injection and axillary lymphadenitis. However, there was no relationship between vaccine dose and tuberculin reactions 1 year later. Ashley and Siebenmann [3] examined the effect of high-dose versus low-dose vaccine (Connaught BCG) and found statistically but not clinically significant differences in tuberculin reactions. In a third study [4], neonates who were aged 7–21 days and who received twice the usual dose of BCG (0.1 mg of Connaught BCG) had more-frequent local reactions than did neonates who received one-fourth of the usual dose (0.125 mg); however, 8 weeks later, tuberculin reactivity was not different in the two groups.
In one study in which the effect of administration technique was examined, the multipuncture technique resulted in significantly fewer conversions to positive tuberculin skin tests than did the intradermal method [5].
What Factors Affect the Waning of Tuberculin Reactivity after BCG Vaccination?
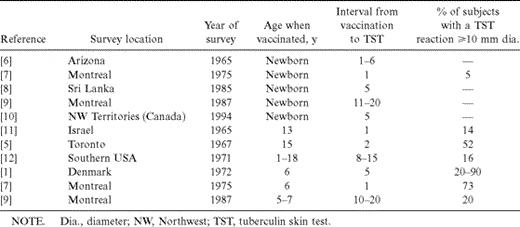
There is consistent evidence (summarized in table 1) [1,–12] that tuberculin reactivity after BCG vaccination in infancy wanes rapidly in all individuals. Tuberculin reactivity after BCG vaccination is primarily affected by age at vaccination. If the vaccine is given in infancy, tuberculin reactions wane rapidly in all individuals. At 6 months or 1 year after vaccination in the neonatal period, none of 104 [7] or 250 [6] infants, respectively, who underwent tuberculin skin testing had reactions ⩾10 mm in diameter. In 1100 Montreal-area schoolchildren and young adults who had received a BCG vaccination in infancy, no effect on tuberculin reactivity could be discerned after an interval of 10–25 years [13]. Similarly, tuberculin reactions in Irish [10] and Sri Lankan [8] children who had received a BCG vaccination in infancy were no different from those in children who had not received a vaccination.
Effect of bacille Calmette-Guérin (BCG) vaccination on initial tuberculin reactions.
On the other hand, a BCG vaccination administered after the first year of life results in persistent tuberculin reactions in a substantial proportion of vaccine recipients. Of Danish schoolchildren who had been vaccinated at age 6 years, most had significant tuberculin reactions 5 years after vaccination [1]. Although the proportion of individuals with persistent reactivity varied according to the manufacturer of the vaccine, 8 of the 10 BCG vaccines tested resulted in persistent tuberculin reactivity in >75% of subjects.
However, in the only study of a large population that received BCG vaccination after the first year of life and was tested >10 years later, persistent tuberculin reactions were much less frequent [9]. Of individuals vaccinated between the ages of 2 and 5 years, 10%–15% had persistent tuberculin reactions, compared with 20%–25% of those vaccinated between the ages of 6 and 10 years. The interval between vaccination and tuberculin testing varied between 10 and 25 years, but the results of multivariate analysis revealed that interval did not have a significant effect after adjusting for age of vaccination. In the same study, repeated vaccination had less effect on the likelihood of subsequent tuberculin reactivity than did age at last vaccination. Similar results were noted in a small study of military recruits who underwent tuberculin skin testing 8–15 years after vaccination [12]. Sepulveda et al. [14, 15] reported similar findings indicating that the prevalence of tuberculin reactions increased in proportion to the number of BCG scars, implying that individuals who were vaccinated at an older age had a greater likelihood of reaction. Unfortunately, in this study, the confounding effects of interval and the number of vaccinations could not be separated from the effect of age at vaccination.
In addition to the differences in the proportion of individuals with tuberculin reactions, the pattern of tuberculin reactions is also different for those who were given BCG vaccine in infancy compared with those who were vaccinated at an older age. The frequency distribution of tuberculin reactions among individuals vaccinated in infancy [6, 7, 9] resembles that seen among patients who have been sensitized to nontuberculous mycobacteria [16, 17] (i.e., the distribution is skewed to the right, and almost all reactions are <10 mm in diameter). However, the distribution of tuberculin reactions among individuals who received BCG vaccination at an older age is bimodal—one mode is centered at zero, and the second mode is centered at 12–15 mm [1, 9], similar to the pattern seen for a population with natural tuberculous infection [18].
In summary, the age at vaccination has a profound influence on the size, pattern, and duration of tuberculin reactions measured ⩾1 year later. The evidence from tuberculin reactions suggests that the immunologic response to BCG vaccination received during infancy is different in terms of the mechanism and/or antigens recognized. It is interesting that BCG vaccination received during infancy has been shown to be highly effective in protecting against the more-severe forms of tuberculosis (i.e., miliary and meningeal disease). For individuals vaccinated after the first year of life, persistence of tuberculin reactivity appears to be an all-or-nothing phenomenon. The majority of these individuals have no reactions, yet a considerable minority have reaction sizes similar to those found in a population with true tuberculous infection.
The “booster phenomenon” is the term given to the increased tuberculin reactions seen when a second skin test is administered 1 week to 1 year after administration of a first skin test that is nonreactive. This phenomenon is believed to represent an anamnestic recall of immune response that occurs in individuals with remote exposure to mycobacterial antigens. Given the evidence of the waning of tuberculin reactions after BCG vaccination, it is not surprising that the booster phenomenon is seen in individuals with remote BCG vaccination. Boosting of tuberculin reactions was first described in 1955 by Magnus and Edwards [19], who noted that the tuberculin reactions of individuals tested 4 years after BCG vaccination were significantly larger if they had been tested annually during the interval than if they had been tested only 4 years after vaccination. The booster phenomenon associated with BCG vaccination was subsequently reported in at least 4 studies [14, 15, 20, 21]. These studies demonstrated that the booster phenomenon was most common in individuals who received vaccination at an older age but that it will continue to be demonstrated for those who received vaccination in infancy after a third skin test was done, similar to the pattern of boosting of tuberculin reactions that is seen in very elderly individuals [22, 23].
What Is the Relationship between Tuberculin Reactivity and Vaccine Efficacy?
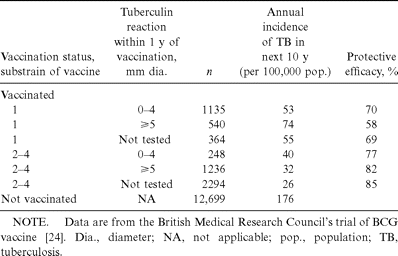
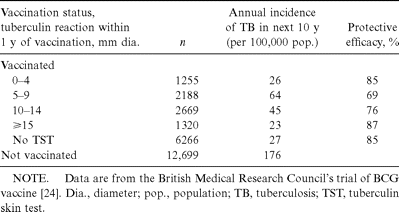
The relationship between vaccine efficacy and postvaccination tuberculin reactivity was examined in the British Medical Research Council's randomized, placebo-controlled trial of BCG vaccination in adolescents in Britain. In this study [11, 24], the findings of which are summarized in tables 2 and 3, 4 different BCG strains were used; 1 of these strains produced tuberculin reactivity in only 30% of vaccine recipients after 8 weeks, whereas the remainder produced tuberculin reactivity in >80% of vaccine recipients. Over the next 15 years, reduction in the incidence of tuberculosis in vaccine recipients relative to that in the placebo group was equivalent for the 4 strains. The incidence of tuberculosis among vaccine recipients grouped by the size of their postvaccination tuberculin reactions (0–4, 5–9, 10–14, and ⩾15 mm) was no different. In both the control subjects and the population groups who received different strains of BCG, there was no difference that might have otherwise explained these differences in tuberculin reactions. Small differences in the protective efficacy between subgroups of vaccine recipients were not statistically significant. The results of this study suggest that the ability of a strain of BCG vaccine to induce tuberculin reactivity is unrelated to the protective efficacy of the vaccine against tuberculosis. The findings are supported by a recent report from Saudi Arabia [25], where the protective efficacy of the BCG vaccine used was high yet very few of the recipients had persistent tuberculin reactivity. A similar conclusion was also reached in an earlier review of the relationship between tuberculin reactions and the efficacy of the BCG vaccine [26].
Relationship of tuberculin reactivity to protective efficacy after vaccination, according to substrain of vaccine.
Relationship of tuberculin reactivity to protective efficacy after bacille Calmette-Guérin (BCG) vaccination, according to size of tuberculin reaction.
In a series of animal studies, Youmans [27] found that tuberculin reactivity had no relationship to immunity. In fact, animals with larger tuberculin reactions after natural infection with M. tuberculosis had a greater risk of subsequent development of the disease. This finding has also been shown for human populations such as US military recruits [28].
In most countries, for a BCG vaccine produced by a particular manufacturer to be licensed, regulatory bodies require that tuberculin skin tests convert to positive for at least 90%–95% of vaccine recipients. Immunogenic activity or protective efficacy does not have to be demonstrated. Therefore, strains that lose their ability to produce tuberculin reactions will be discarded, but there is no such selection effect on strains that lose their immunogenic activity or protective efficacy.
What about Postvaccination Scars and Vaccine Efficacy?
The second most common measure of the effect of BCG vaccination is ascertainment and measurement of scar formation. Unfortunately, in 20%–50% of BCG vaccine recipients, a scar will fail to develop [21, 29]. In addition, although scar formation has been shown to correlate with tuberculin reactions in one study [1], this correlation has not been demonstrated in several other investigations [4, 11, 30].
In case-control studies that classified the status of the BCG vaccine on the basis of postvaccination scars, the range of efficacy of the BCG vaccine was 50%–60% [31]. There may be considerable selection bias in such case-control studies, because individuals who had never received BCG vaccination may have had socioeconomic, health-behavior, or other confounding risk factors that independently increased their risk of tuberculosis. Another possibility is that individuals without scars had in fact been vaccinated but had failed to manifest a sufficient local reaction. In a large survey in sub-Saharan Africa, the incidence of tuberculosis was virtually identical among individuals with and without scars after BCG vaccination [32].
Conclusions
The protective efficacy of BCG vaccine is excellent for infants but is less so for older children and adults. The results of studies of tuberculin reactivity suggest that the immune response to the BCG vaccine is different in infancy than in childhood. Postvaccination tuberculin reactivity is not an indicator of protective efficacy of BCG vaccination, because it is not an indicator of immunity to M. tuberculosis. The ideal BCG vaccine would stimulate the development of effective immunity yet would have no effect on tuberculin reactivity.
References
Author notes
Financial support: D.M. was supported by a Chercheur Clinicien (Clinician Scientist) award from Fonds de Recherche en Santé du Québec.





Comments