-
PDF
- Split View
-
Views
-
Cite
Cite
Shelley A. Gilroy, Mary A. M. Rogers, Donald C. Blair, Treatment of Latent Tuberculosis Infection in Patients Aged ≥35 Years, Clinical Infectious Diseases, Volume 31, Issue 3, September 2000, Pages 826–829, https://doi.org/10.1086/314037
Close - Share Icon Share
Abstract
Treating patients aged ≥35 years for tuberculosis infection has been controversial because of the hepatotoxic effects of isoniazid. A 2-year retrospective cohort study of outpatient medical records determined the completion rate in this age group and identified risk factors associated with isoniazid-associated hepatotoxicity. Isoniazid preventative therapy was well tolerated. However, toxicity occurred in women receiving concomitant medications and men who used alcohol.
In the mid-1960s, when isoniazid was found to be effective in the chemoprophylaxis of tuberculosis, its use was advocated for all persons with positive skin tests [1]. The incidence of serious toxicity was thought to be ≤0.2% [2]. Later, reports of serial liver function studies of patients receiving isoniazid prophylaxis showed that the drug did frequently produce subclinical and clinical hepatitis [3–6]. These reports led to the recommendation of restricting the use of isoniazid prophylaxis to those reactive to tuberculin who were aged <35 years or those reactive to tuberculin who were aged ≥35 years and who were at increased risk of activation [7]. A 1983 revision of the American Thoracic Society guidelines recommended that clinicians evaluate and periodically monitor liver function and discontinue treatment if aminotransferase levels exceed 3–5 times normal values [8]. Investigators believed that implementation of these guidelines would almost eliminate the fatal hepatotoxicity related to isoniazid prophylaxis [9]. More recently, the Centers for Disease Control and Prevention (CDC) has recommended prophylaxis in recent converters of all ages who are at risk for activation of tuberculosis [10]. Despite CDC recommendations, general internists and family practitioners in our area were still requesting infectious disease consultations regarding the safety of isoniazid for this age group. Therefore, the following retrospective cohort analysis was undertaken to determine the frequency with which adult patients aged ≥35 years completed a 6-month course of preventive therapy and to examine the factors that predicted clinically significant hepatotoxicity and ability to complete therapy.
A retrospective chart review was done for all 510 patients aged ≥35 years with documented reaction to purified protein derivative (PPD) of >10 mm induration who were evaluated at the Onondaga County Health Department tuberculosis clinic in Syracuse, New York, between March 1994 and March 1996. The majority of adults with positive PPD results from Onondaga County are seen in this clinic, and most are from high-risk groups: homeless shelters, refugee clinics, health care facility employees, contacts of active cases, and inmates of correctional facilities. A complete medical history was obtained from each patient by a nurse, including a symptom review and detailed questions about drug allergies, current medications, and use of alcohol. Alcohol use was defined as consuming >3 alcoholic beverages daily; a standardized instrument designed for the measurement of alcohol consumption was not used. A chest radiograph was done, and baseline alanine aminotransferase (ALT) levels were determined. ALT levels were the only liver function indicators monitored, after a previous chart review of treated patients with isoniazid revealed that the ALT level correlated well with significant rises in other liver function indicators (S. A. Gilroy and D. C. Blair, unpublished data).
Patients in high-risk groups were offered isoniazid preventive therapy according to CDC guidelines [10]. The patients were informed of the benefits of isoniazid therapy and of the risks of isoniazid-associated hepatitis (including the symptoms of nausea, abdominal pain, anorexia, and fever), as well as other isoniazid-associated adverse effects such as rash and paresthesias. The necessity for at least monthly symptom review and ALT measurement was emphasized. The importance of abstinence from alcohol while taking isoniazid was stressed. A 1-month supply of isoniazid, 300 mg, plus pyridoxine, 50 mg, was distributed to all patients who consented to preventive therapy. Patients were required to return to the clinic monthly for symptom review, physical examination if indicated, venipuncture for determination of ALT level, and to obtain the next month's medication. A patient was deemed compliant if he or she kept the monthly appointment and denied missed doses when questioned by the clinic nurse. Pill counts and urine testing were not done. The end point was completion of 6 months of therapy with isoniazid. Isoniazid was discontinued if the patient developed symptoms characteristic of isoniazid toxicity or if the ALT level rose to 3–5 times baseline [8]. The ALT level was then monitored, and isoniazid was restarted after the level reverted back to baseline and symptoms resolved, if the patient accepted a second trial of isoniazid. Patients for whom restarting isoniazid resulted in an adverse event such as a second rise in ALT or an allergic reaction and those who declined a second trial of isoniazid were offered rifampin.
Unpaired t tests (for continuous variables) and χ2 tests (for discrete variables) were used to compare participants with nonparticipants and to compare patient characteristics with isoniazid completion. For those tables with at least 1 cell with an expected frequency of <5 patients, exact tests were used. To evaluate dose response for the number of medications, a χ2 test for linear trend was calculated. Type of medication was categorized into the following groups for analysis: antacids, antibiotics, antiepileptics, antihistamines, anti-hypertensives, anti-psychotics/antidepressants, analgesics, broncho-dilators, hor-mones, hypoglycemics, and lipid-lowering agents. One-way analysis of variance (ANOVA) was used for the comparison of mean ALT concentrations in the 3 study groups: subjects who completed isoniazid, subjects who did not complete isoniazid, and subjects who did not complete isoniazid but received rifampin. A final multivariable analysis was conducted by use of nominal logistic regression to assess indicators of isoniazid completion (completed/did not complete/completed with rifampin).
A total of 520 patients met entry criteria, but charts for 10 were unavailable. Of the 510 patients offered preventive therapy, 10 refused treatment, and 165 accepted therapy but were lost to follow-up after initiation of therapy. The most common reasons for refusing therapy were fear of developing isoniazid-associated hepatitis, inability to commit to abstinence from alcohol, and a history of BCG vaccination during childhood. Patients who consented to begin therapy and who were given a 1-month supply of isoniazid and pyridoxine but failed to keep their next month's appointment for medication and clinical assessment were defined as lost to follow-up. Contact by tele-phone and/or registered letter to this group of patients was unsuccessful.
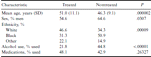
A total of 335 patients started isoniazid preventive therapy: 183 men and 152 women. There were 156 white patients, 105 African American patients, and 74 patients of other ethnicity (Asian, Native American, and Hispanic).
The 335 patients who began treatment with isoniazid (designated as treated) were older than the 175 who did not take isoniazid (designated as untreated). The mean age was 51.0 years (SD, 11.1 years) versus 46.3 years (SD, 9.1 years; P = .000002; table 1). Sex, ethnicity, and alcohol use were also statistically significantly different between patients who agreed to begin preventive therapy (table 1) and those who rejected therapy. Thus, those who were not treated were more likely to be younger, male, black, and users of alcohol.
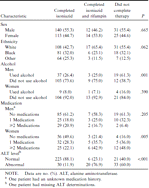
Two hundred fifty-three patients (76%) completed 6 months of isoniazid with minimal or no side effects (table 2). Of these, only 30 (11.9%) developed elevated ALT levels. The most affected patient was a man whose ALT level was 5 times the upper limit of normal and who had symptoms of nausea and jaundice. Isoniazid therapy was interrupted; when resumed after 1 month, the isoniazid was well tolerated, and the patient's ALT level did not rise again. The other 29 patients had ALT elevations of 2–3 times baseline between the third and fourth month of therapy, which subsequently returned to baseline after completion of therapy. Five of these 29 patients described nausea and/or fatigue, but interruption of therapy was not necessary; they were closely monitored clinically, and ALT levels were determined every 2 weeks until ALT levels stabilized and symptoms resolved.
Eighty-two patients (24%) were unable to complete isoniazid therapy because of adverse signs or symptoms, with or without a rise in ALT level (table 2); 1 traumatic death occurred, unrelated to isoniazid therapy. All possible combinations of symptoms and ALT elevation were seen: elevated ALT level and symptoms (49%), elevated ALT level but no symptoms (17%), and normal ALT level and symptoms (28%). The record gave no explanation for discontinuing therapy for 5%.
All 81 surviving patients were offered daily rifampin, but 54 refused further treatment, largely because they were fearful of further side effects. Of the 27 patients who began rifampin, only 1 stopped treatment (abdominal pain), and 26 completed a total of 6 months of therapy. Three factors were associated with failure to complete therapy: use of alcohol by men, use of concomitant medications by women, and occurrence of ele-vated ALT levels (table 2). Of the 22 men who used alcohol and who were unable to complete isoniazid therapy (table 2), 12 (56%) had elevated ALT levels and adverse effects (nausea, jaundice, fatigue, joint aches, and/or rash), 3 (14%) had elevated ALT levels without symptoms, 4 (18%) had normal ALT levels and symptoms (fatigue and/or joint aches), and 3 (14%) had normal ALT levels without symptoms. The ALT elevation was 3–5 times baseline. Eight of the 37 men completed therapy despite using alcohol but with ALT elevations 2–3 times baseline. Two of these 8 men described fatigue; otherwise the group was asymptomatic, was monitored, and did not require interruption of therapy.
For women, there was no correlation between alcohol use and failure to complete isoniazid therapy. There was, however, a direct correlation between the number of concurrent medications used and the likelihood of failure to complete therapy: Women who took 1 medication were 3.5 times as likely not to complete isoniazid therapy as women who took no medication; women who took ≥2 drugs were 5.8 times as likely not to complete the therapy (χ2 for linear trend, 13.1; P = .00029).
Despite the small numbers, we explored the question of whether the therapeutic class of medication used was associated with failure to complete isoniazid therapy (table 3). Of the 11 different types of medications used by our patients, there were suggested associations between isoniazid completion and 3 types of drugs: antipsychotics and antidepressants (P=.083), lipid-lowering agents (P=.025), and antihistamines (P = .063). Drugs were grouped by pharmacologic activity. Of the 22 patients who took antipsychotics or antidepressants, 15 (68%) completed the isoniazid regimen, compared with an 84% completion rate by those not taking those drugs. Although only 6 patients took lipid-lowering agents, only 2 (33%) completed isoniazid therapy, which contrasts sharply with the 76% completion rate for the subjects not taking these medications. Similarly, the isoniazid completion rate for the 6 patients taking antihistamines was 50%, with a 76% rate for patients not receiving antihistamines. Hormones, antacids, antihypertensives, bronchodilators, analgesics, hypoglycemics, antibiotics, and anti-epileptic medications did not significantly affect completion of isoniazid therapy.
ALT levels were significantly associated with completion of isoniazid therapy (P < .001). Although 91% of those patients with ALT levels that remained within the normal range completed isoniazid or isoniazid followed by rifampin, only 60.2% of those with ALT levels outside the normal range did so. The mean ALT level of patients who completed isoniazid therapy was considerably lower (27.1 U/L) than that for patients who did not (95.6 U/L) or for patients who received rifampin (88 U/L; data not shown). These differences were statistically significant (P < .000001, ANOVA).
It should be emphasized that an elevated ALT level does not necessarily mean that therapy needs to be terminated. Of the 253 patients who completed the entire regimen of isoniazid, 30 had ALT levels outside the normal range but were still able to complete the therapy. These data also suggest that patients who were taking concomitant medications were more likely to have elevated ALT levels (P = .09287; data not shown). As expected, patients with elevated ALT levels were more likely to exhibit symptoms (P < .001), which was true for both men and women and for both alcohol users and nonusers. Patients with abnormal ALT levels were 9.52 times as likely to report symptoms as were patients with normal ALT levels (table 4). Four patients had missing information regarding ALT levels and/or symptoms. The unrelated death was a patient who was missing an ALT determination, and 3 patients who completed therapy were missing information regarding symptoms.
Age (data not shown), ethnicity, sex, alcohol use by women, and concomitant medication in men were not statistically significantly different among the 3 groups (table 2). Multivariate analysis was conducted by use of nominal logistic regression to assess combined effect of the patient characteristics as listed in table 2 and isoniazid completion (completed/completed isoniazid and rifampin/did not complete). Only ALT level was statistically significant (P < .0001) after adjustment for the other variables.
In this population of subjects, age had no effect on whether the patient was able to complete therapy with isoniazid.
The association of the use of concomitant medications by women with failure to complete therapy appears not to have been described elsewhere. Others have shown that older women are less likely to complete preventive therapy [11, 12], and this has been linked to the occurrence of elevated results of liver function tests [11–14]. None of these studies mentions whether the women were taking concomitant medications. It is not clear whether failure to complete isoniazid therapy was because of the concurrent medications or the medications are markers for other determinants of incomplete therapy, such as inability to comply with follow-up.
It is well established that alcohol use while taking isoniazid increases the incidence of hepatitis, although the mechanism is unclear. The risk of isoniazid-associated hepatitis is increased also in alcoholics with preexisting liver abnormalities. Alcohol use in this cohort greatly affected whether the patient was able to complete preventive therapy with isoniazid. The majority of the men in our study who used alcohol and who were unable to complete therapy with isoniazid developed ALT elevations 5 times baseline, many with symptoms. Once therapy was initiated (i.e., the patient came back for the first month's evaluation and dispensing of medication), the interaction between the alcohol and isoniazid, not behavioral factors, led to withdrawal of the isoniazid.
An interesting association between taking certain types of medications and their impact on whether a patient was able to complete isoniazid therapy was noted. Although the numbers were small, patients who were taking isoniazid as well as an antipsychotic or antidepressant medication, a lipid-lowering agent, or antihistamine were less likely to complete preventive therapy with isoniazid. This occurred in both men and women. All patients who took antipsychotic or antidepressant medications in addition to isoniazid and who were unable to complete therapy developed side effects and or ALT elevation, suggesting drug interaction rather than a behavioral influence on completion of therapy.
An inherent problem in this review is the assumption that all patients were compliant with the preventive therapy treatment regimen. Compliance of patients is difficult to measure unless the medication is administered under directly observed therapy. Patients who begin preventive therapy at Onondaga County Health Department are required to keep monthly appointments for ALT level and symptom monitoring but do not receive directly observed therapy. During the time of this retro-spective study, health care workers relied on the patients' account of pill taking. Pill counts and urine testing were not done. Poor compliance could explain lack of side effects or rise in ALT levels. However, we think it unlikely that these patients would comply with their scheduled monthly visits but not with their daily medication. Although we did not perform pill counts (well known not to be a reliable check of compliance), we did pay careful attention to the interval since the previous visit; the majority of these patient visits were on schedule.
In this retrospective study, patients' history of alcohol use was obtained by use of standard medical history techniques. Although this study did not address the issue of a possible linear or other correlation of the amount of alcohol with completion of isoniazid therapy, this would be a good focus for a prospective study, with use of a standardized instrument for measuring the amount of alcohol consumed.
These data show that the use of isoniazid by patients aged ≥35 years is safe and well tolerated when monthly symptom and ALT monitoring are used. Groups of patients at particular risk of toxicity are women receiving concomitant medications and male alcohol users. Most of the patients who stopped isoniazid but completed their 6-month course with rifampin had no side effects, suggesting this could be a well-tolerated alternative in this subset of patients. Further studies are necessary to establish whether certain concomitant medications, such as lipid-lowering agents, antipsychotic and antidepressant medications, or antihistamines, increase the risk of developing hepatotoxicity from isoniazid.
The toxicity of isoniazid in persons aged >35 years can readily be managed in the setting of an adequately staffed county tuberculosis clinic, through a program of meticulous monthly (or more frequent, if indicated) follow-up of symptoms and one simple liver function test. The use of isoniazid for the treatment of latent tuberculosis infection should routinely be extended to include those older higher-risk persons for whom regular monitoring is feasible.
Acknowledgments
We thank Kaye Barrington and Sally Melton for secretarial assistance, Colleen Cunningham and Reid Muller for manuscript review, and the staff at the Onondaga County Health Department Tuberculosis Clinic for chart retrievals.
References
Figures and Tables
Comparison between patients who began preventive therapy for latent tuberculosis infection (n = 335) and those who did not (n = 175).
Characteristics of patients who completed and did not complete preventive therapy for latent tuberculosis infection.
Medication use by patients who completed and did not complete preventive therapy for latent tuberculosis infection.
Presence of symptoms by level of alanine aminotransferase (ALT) among patients with latent tuberculosis infection.







Comments