Abstract
The clinical efficacy of bariatric surgery has encouraged the scientific investigation of the gut as a major endocrine organ. Manipulation of gastrointestinal anatomy through surgery has been shown to profoundly affect the physiological and metabolic processes that control body weight and glycaemia. The most popular bariatric surgical procedures are gastric bypass, adjustable gastric banding and vertical sleeve gastrectomy. Even though these procedures were designed with the aim of causing restriction of food intake and nutrient malabsorption, evidence suggests that their contributions to weight loss are minimal. Instead, these interventions reduce body weight by decreasing hunger, increasing satiation during a meal, changing food preferences and energy expenditure. In this Review, we have explored these mechanisms as well as their mediators. The hope is that that their in-depth investigation will enable the optimization and individualization of surgical techniques, the development of equally effective but safer nonsurgical weight-loss interventions, and even the understanding of the pathophysiology of obesity itself.
Key Points
-
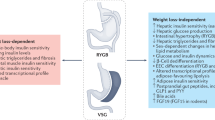
Bariatric surgery is the most effective treatment for weight loss and its long-term maintenance; the most commonly performed procedures are laparoscopic gastric bypass, adjustable gastric banding and vertical sleeve gastrectomy
-
Bariatric surgery improves obesity-related comorbidities and reduces overall and cardiovascular mortality
-
Gastric bypass works by reducing hunger, increasing satiation, changing food preferences and increasing diet-induced energy expenditure
-
Adjustable gastric banding works probably through the reduction in hunger, which might be mediated through vagal signalling
-
Some of the clinical and physiological effects of vertical sleeve gastrectomy are similar to gastric bypass
-
Understanding the mechanisms of action of these procedures could accelerate their optimization and the development of novel, and hopefully safer, medications for obesity and type 2 diabetes mellitus
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Olbers, T., Lonroth, H., Fagevik-Olsen, M. & Lundell, L. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes. Surg. 13, 364–370 (2003).
Burton, P. R. & Brown, W. A. The mechanism of weight loss with laparoscopic adjustable gastric banding: induction of satiety not restriction. Int. J. Obes. (Lond) 35 (Suppl. 3), S26–S30 (2011).
Scopinaro, N. Thirty-five years of biliopancreatic diversion: notes on gastrointestinal physiology to complete the published information useful for a better understanding and clinical use of the operation. Obes. Surg. 22, 427–432 (2012).
Carlin, A. M. et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann. Surg. 257, 791–797 (2013).
O'Brien, P. E., McPhail, T., Chaston, T. B. & Dixon, J. B. Systematic review of medium-term weight loss after bariatric operations. Obes. Surg. 16, 1032–1040 (2006).
Brethauer, S. A., Hammel, J. P. & Schauer, P. R. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg. Obes. Relat. Dis. 5, 469–475 (2009).
Vest, A. R., Heneghan, H. M., Agarwal, S., Schauer, P. R. & Young, J. B. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 98, 1763–1777 (2012).
Pontiroli, A. E. & Morabito, A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann. Surg. 253, 484–487 (2011).
Buchwald, H. et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am. J. Med. 122, 248–256.e245 (2009).
Flum, D. R. et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 361, 445–454 (2009).
Maclean, P. S., Bergouignan, A., Cornier, M. A. & Jackman, M. R. Biology's response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R581–R600 (2011).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Hofmann, W., van Koningsbruggen, G. M., Stroebe, W., Ramanathan, S. & Aarts, H. As pleasure unfolds. Hedonic responses to tempting food. Psychol. Sci. 21, 1863–1870 (2010).
le Roux, C. W. et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 243, 108–114 (2006).
Dixon, A. F., Dixon, J. B. & O'Brien, P. E. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J. Clin. Endocrinol. Metab. 90, 813–819 (2005).
Miras, A. D. et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 96, 467–473 (2012).
Wilson-Perez, H. E. et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int. J. Obes. (Lond.) 37, 288–295 (2012).
Stefater, M. A. et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138, 2426–2436 (2010).
Topart, P., Becouarn, G. & Ritz, P. Pouch size after gastric bypass does not correlate with weight loss outcome. Obes. Surg. 21, 1350–1354 (2011).
Guijarro, A. et al. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1474–R1489 (2007).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Hatoum, I. J. et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 97, E1023–E1031 (2012).
Faulconbridge, L. F. et al. Changes in symptoms of depression with weight loss: results of a randomized trial. Obesity (Silver Spring) 17, 1009–1016 (2009).
Korner, J. et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 14, 1553–1561 (2006).
Karamanakos, S. N., Vagenas, K., Kalfarentzos, F. & Alexandrides, T. K. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann. Surg. 247, 401–407 (2008).
Batterham, R. L. et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nature 418, 650–654 (2002).
Sloth, B., Holst, J. J., Flint, A., Gregersen, N. T. & Astrup, A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am. J. Physiol. Endocrinol. Metab. 292, E1062–E1068 (2007).
Meguid, M. M., Glade, M. J. & Middleton, F. A. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition 24, 832–842 (2008).
Dirksen, C. et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. (Lond.) http://dx.doi.org/10.1038/ijo.2013.15.
Chandarana, K. et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60, 810–818 (2011).
Peterli, R. et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann. Surg. 250, 234–241 (2009).
Larsen, P. J., Tang-Christensen, M., Holst, J. J. & Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270 (1997).
Suzuki, K., Jayasena, C. N. & Bloom, S. R. Obesity and appetite control. Exp. Diabetes Res. 2012, 824305 (2012).
Russell-Jones, D. & Gough, S. Recent advances in incretin-based therapies. Clin. Endocrinol. (Oxf.) 77, 489–499 (2012).
Wilson-Perez, H. E. et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes http://dx.doi.org/10.2337/db12-1498.
Dirksen, C. et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol. Motil. 25, 346–e255 (2013).
Shah, S. et al. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg. Obes. Relat. Dis. 6, 152–157 (2010).
Roberge, J. N. & Brubaker, P. L. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 133, 233–240 (1993).
Dar, M. S. et al. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes. Surg. 22, 1077–1083 (2012).
Date, Y. et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141, 4255–4261 (2000).
Romero, A. et al. GOAT: the master switch for the ghrelin system? Eur. J. Endocrinol. 163, 1–8 (2010).
Cummings, D. E. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001).
Wang, L., Saint-Pierre, D. H. & Tache, Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y—synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 325, 47–51 (2002).
Lawrence, C. B., Snape, A. C., Baudoin, F. M. & Luckman, S. M. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143, 155–162 (2002).
Dimitriadis, E. et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann. Surg. 257, 647–654 (2013).
Cummings, D. E. et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 346, 1623–1630 (2002).
Barazzoni, R. et al. Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity (Silver Spring) http://dx.doi.org/10.1038/oby.2012.149.
Espelund, U., Hansen, T. K., Orskov, H. & Frystyk, J. Assessment of ghrelin. APMIS Suppl. 109, 140–145 (2003).
Chambers, A. P. et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144, 50–52.e5 (2012).
Korner, J. et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int. J. Obes. (Lond.) 33, 786–795 (2009).
Gribble, F. M. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc. Nutr. Soc. 71, 456–462 (2012).
Seyfried, F., le Roux, C. W. & Bueter, M. Lessons learned from gastric bypass operations in rats. Obes. Facts 4, (Suppl. 1), 3–12 (2011).
Bjorklund, P. et al. Is the Roux limb a determinant for meal size after gastric bypass surgery? Obes. Surg. 20, 1408–1414 (2010).
Shin, A. C., Zheng, H. & Berthoud, H. R. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann. Surg. 255, 294–301 (2012).
Kampe, J. et al. Neural and humoral changes associated with the adjustable gastric band: insights from a rodent model. Int. J. Obes. (Lond.) 36, 1403–1411 (2012).
Burton, P. R., Brown, W. A., Laurie, C., Hebbard, G. & O'Brien, P. E. Mechanisms of bolus clearance in patients with laparoscopic adjustable gastric bands. Obes. Surg. 20, 1265–1272 (2010).
de Jong, J. R., van Ramshorst, B., Gooszen, H. G., Smout, A. J. & Tiel- Van Buul, M. M. Weight loss after laparoscopic adjustable gastric banding is not caused by altered gastric emptying. Obes. Surg. 19, 287–292 (2009).
Burton, P. R. et al. Effects of adjustable gastric bands on gastric emptying, supra- and infraband transit and satiety: a randomized double-blind crossover trial using a new technique of band visualization. Obes. Surg. 20, 1690–1697 (2010).
Burton, P. R. et al. Effects of gastric band adjustments on intraluminal pressure. Obes. Surg. 19, 1508–1514 (2009).
Heneghan, H. M., Yimcharoen, P., Brethauer, S. A., Kroh, M. & Chand, B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg. Obes. Relat. Dis. 8, 408–415 (2012).
Campos, G. M. et al. Factors associated with weight loss after gastric bypass. Arch. Surg. 143, 877–883 (2008).
Bueter, M. et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes. Surg. 20, 616–622 (2010).
Madan, A. K., Tichansky, D. S. & Phillips, J. C. Does pouch size matter? Obes. Surg. 17, 317–320 (2007).
Braghetto, I. et al. Evaluation of the radiological gastric capacity and evolution of the BMI 2–3 years after sleeve gastrectomy. Obes. Surg. 19, 1262–1269 (2009).
Deguines, J. B. et al. Is the residual gastric volume after laparoscopic sleeve gastrectomy an objective criterion for adapting the treatment strategy after failure? Surg. Obes. Relat. Dis. http://dx.doi.org/10.1016/j.soard.2012.11.010.
Pomerri, F. et al. Laparoscopic sleeve gastrectomy--radiological assessment of fundus size and sleeve voiding. Obes. Surg. 21, 858–863 (2011).
Langer, F. B. et al. Does gastric dilatation limit the success of sleeve gastrectomy as a sole operation for morbid obesity? Obes. Surg. 16, 166–171 (2006).
Wang, G. et al. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes. Surg. 22, 1263–1267 (2012).
Suzuki, S., Ramos, E. J., Goncalves, C. G., Chen, C. & Meguid, M. M. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 138, 283–290 (2005).
Naslund, I. & Beckman, K. W. Gastric emptying rate after gastric bypass and gastroplasty. Scand. J. Gastroenterol. 22, 193–201 (1987).
Horowitz, M., Collins, P. J., Harding, P. E. & Shearman, D. J. Gastric emptying after gastric bypass. Int. J. Obes. 10, 117–121 (1986).
Melissas, J. et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. http://dx.doi.org/10.1097/SLA.0b013e3182774522.
Braghetto, I. et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes. Surg. 19, 1515–1521 (2009).
Melissas, J. et al. Sleeve gastrectomy: a restrictive procedure? Obes. Surg. 17, 57–62 (2007).
Bernstine, H. et al. Gastric emptying is not affected by sleeve gastrectomy—scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum. Obes. Surg. 19, 293–298 (2009).
Kumar, R. et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 149, 654–661 (2011).
Odstrcil, E. A. et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 92, 704–713 (2010).
Shin, A. C. et al. Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats. Obes. Surg. 23, 531–540 (2012).
Saeidi, N. et al. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int. J. Obes. (Lond.) 36, 1396–1402 (2012).
Stylopoulos, N., Hoppin, A. G. & Kaplan, L. M. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 17, 1839–1847 (2009).
Cornicelli, M., Noli, G., Marinari, G. M. & Adami, G. F. Dietary habits and body weight at long-term following biliopancreatic diversion. Obes. Surg. 20, 1278–1280 (2010).
Patti, M. E. et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 17, 1671–1677 (2009).
Pournaras, D. J. et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153, 3613–3619 (2012).
Simonen, M. et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes. Surg. 22, 1473–1480 (2012).
Kohli, R. et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J. Clin. Endocrinol. Metab. 98, E708–E712 (2013).
Stefater, M. A. et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141, 939–949 (2011).
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J. & Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 (2008).
Ryan, K. K. et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Sarruf, D. A. et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–1824 (2010).
Ogundare, M. et al. Cerebrospinal fluid steroidomics: are bioactive bile acids present in brain? J. Biol. Chem. 285, 4666–4679 (2010).
Keitel, V. et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 (2010).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Zhang, H. et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl Acad. Sci. USA 106, 2365–2370 (2009).
Furet, J. P. et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057 (2010).
Li, J. V. et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 60, 1214–1223 (2011).
Liou, A. P. et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci.Transl. Med. 5, 178ra141 (2013).
Kenler, H. A., Brolin, R. E. & Cody, R. P. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 52, 87–92 (1990).
Olbers, T. et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann. Surg. 244, 715–722 (2006).
Ernst, B., Thurnheer, M., Wilms, B. & Schultes, B. Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes. Surg. 19, 274–280 (2009).
Mathes, C. M. & Spector, A. C. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol. Behav. 107, 476–483 (2012).
Zheng, H. et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1273–R1282 (2009).
Bueter, M. et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 104, 709–721 (2011).
Ochner, C. N. et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 253, 502–507 (2011).
Ochner, C. N. et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 209, 128–135 (2012).
Ochner, C. N. et al. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci. Res. 74, 138–143 (2012).
Scholtz S. et al. Obese patients after gastric bypass surgery have lower brain hedonic responses to food than after gastric banding. Gut (in press).
Dunn, J. P. et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 1350, 123–130 (2010).
Steele, K. E. et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 20, 369–374 (2010).
Shin, A. C., Zheng, H., Pistell, P. J. & Berthoud, H. R. Roux-en-Y gastric bypass surgery changes food reward in rats. Int. J. Obes. (Lond.) 35, 642–651 (2010).
Shin, Y. K. et al. Modulation of taste sensitivity by GLP-1 signaling. J. Neurochem. 106, 455–463 (2008).
De Silva, A. et al. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 14, 700–706 (2011).
Thiele, T. E. et al. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am. J. Physiol. 272, R726–R730 (1997).
Halatchev, I. G. & Cone, R. D. Peripheral administration of PYY3–36 produces conditioned taste aversion in mice. Cell Metab. 1, 159–168 (2005).
Mathes, C. M. et al. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R751–R767 (2011).
Tack, J., Arts, J., Caenepeel, P., De Wulf, D. & Bisschops, R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat. Rev. Gastroenterol. Hepatol. 6, 583–590 (2009).
Mallory, G. N., Macgregor, A. M. & Rand, C. S. The influence of dumping on weight loss after gastric restrictive surgery for morbid obesity. Obes. Surg. 6, 474–478 (1996).
le Roux, C. W. et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1057–R1066 (2011).
Tamboli, R. A. et al. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 18, 1718–1724 (2010).
Carrasco, F. et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes. Surg. 17, 608–616 (2007).
Bobbioni-Harsch, E. et al. Energy economy hampers body weight loss after gastric bypass. J. Clin. Endocrinol. Metab. 85, 4695–4700 (2000).
Das, S. K. et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am. J. Clin. Nutr. 78, 22–30 (2003).
Flancbaum, L., Choban, P. S., Bradley, L. R. & Burge, J. C. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery 122, 943–949 (1997).
Busetto, L. et al. Relationship between energy expenditure and visceral fat accumulation in obese women submitted to adjustable silicone gastric banding (ASGB). Int. J. Obes. Relat. Metab. Disord. 19, 227–233 (1995).
Galtier, F. et al. Resting energy expenditure and fuel metabolism following laparoscopic adjustable gastric banding in severely obese women: relationships with excess weight lost. Int. J. Obes. (Lond.) 30, 1104–1110 (2006).
Faria, S. L., Faria, O. P., Cardeal Mde, A., de Gouvea, H. R. & Buffington, C. Diet-induced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 8, 797–802 (2012).
Werling, M. et al. Increased postprandial energy expenditure may explain superior long term weight loss after Roux-en-Y gastric bypass compared to vertical banded gastroplasty. PLoS ONE 8, e60280 (2013).
Bueter, M. et al. Gastric bypass increases energy expenditure in rats. Gastroenterology 138, 1845–1853 (2010).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Hankir, M. et al. Increased energy expenditure in gastric bypass rats is not caused by activated brown adipose tissue. Obes. Facts 5, 349–358 (2012).
Gersin, K. S. et al. Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest. Endosc. 71, 976–982 (2010).
Acknowledgements
A. D. Miras is funded by the Medical Research Council (MRC) Research Training Fellowship G0902002 and a MRC Research Career Development Centenary Award. C. W. le Roux is funded by the Science Foundation Ireland and the Moulton Foundation.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Miras, A., le Roux, C. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 10, 575–584 (2013). https://doi.org/10.1038/nrgastro.2013.119
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.119
This article is cited by
-
Leptin receptor deficiency impedes metabolic surgery related-weight loss through inhibition of energy expenditure in db/db mice
Diabetology & Metabolic Syndrome (2024)
-
The effect of bariatric surgery on the expression of gastrointestinal taste receptors: A systematic review
Reviews in Endocrine and Metabolic Disorders (2024)
-
HSP47 levels determine the degree of body adiposity
Nature Communications (2023)
-
Hypoabsorptive surgeries cause limb-dependent changes in the gut endocannabinoidome and microbiome in association with beneficial metabolic effects
International Journal of Obesity (2023)
-
The impact of maternal bariatric surgery on long-term health of offspring: a scoping review
Pediatric Research (2023)