Abstract
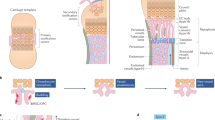
Bone never forms without vascular interactions. This simple statement of fact does not adequately reflect the physiological and pharmacological implications of the relationship. The vasculature is the conduit for nutrient exchange between bone and the rest of the body. The vasculature provides the sustentacular niche for development of osteoblast progenitors and is the conduit for egress of bone marrow cell products arising, in turn, from the osteoblast-dependent haematopoietic niche. Importantly, the second most calcified structure in humans after the skeleton is the vasculature. Once considered a passive process of dead and dying cells, vascular calcification has emerged as an actively regulated form of tissue biomineralization. Skeletal morphogens and osteochondrogenic transcription factors are expressed by cells within the vessel wall, which regulates the deposition of vascular calcium. Osteotropic hormones, including parathyroid hormone, regulate both vascular and skeletal mineralization. Cellular, endocrine and metabolic signals that flow bidirectionally between the vasculature and bone are necessary for both bone health and vascular health. Dysmetabolic states including diabetes mellitus, uraemia and hyperlipidaemia perturb the bone–vascular axis, giving rise to devastating vascular and skeletal disease. A detailed understanding of bone–vascular interactions is necessary to address the unmet clinical needs of an increasingly aged and dysmetabolic population.
Key Points
-
Clinically important and actively regulated processes control tissue mineralization in the skeleton and the arterial vasculature
-
Bidirectional communication between the vasculature and bone—conveyed by cellular, endocrine and metabolic messengers—is critical to maintenance of bone health and vascular health
-
Dysmetabolic states, such as diabetes mellitus, uraemia and hyperlipidaemia, perturb the bone–vascular axis and give rise to vascular and skeletal disease
-
As understanding of the bone–vascular axis continues to improves, so too will our capacity to meet the clinical needs of patients with metabolic bone and cardiovascular disorders
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zelzer, E. et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129, 1893–1904 (2002).
Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010).
Qing, H. et al. PTHR1 in osteocytes plays a major role in perilacunar remodeling through the activation of “osteoclastic” genes in osteocytes [abstract]. J. Bone Miner. Res. 25 (Suppl. 1), a1082 (2010).
Reeve, J. et al. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rate. J. Clin. Endocrinol. Metab. 66, 1124–1131 (1988).
Bianco, P. Bone and the hematopoietic niche: a tale of two stem cells. Blood 117, 5281–5288 (2011).
Eash, K. J., Greenbaum, A. M., Gopalan, P. K. & Link, D. C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120, 2423–2431 (2010).
Towler, D. A. The osteogenic-angiogenic interface: novel insights into the biology of bone formation and fracture repair. Curr. Osteoporos. Rep. 6, 67–71 (2008).
Collins, T. C. et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation 119, 2305–2312 (2009).
Guzman, R. J. et al. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J. Am. Coll. Cardiol. 51, 1967–1974 (2008).
Soor, G. S., Vukin, I., Leong, S. W., Oreopoulos, G. & Butany, J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology 40, 385–391 (2008).
Everhart, J. E., Pettitt, D. J., Knowler, W. C., Rose, F. A. & Bennett, P. H. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia 31, 16–23 (1988).
Lehto, S., Niskanen, L., Suhonen, M., Rönnemaa, T. & Laakso, M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 16, 978–983 (1996).
Napoli, C. et al. Therapeutic targeting of the stem cell niche in experimental hindlimb ischemia. Nat. Clin. Pract. Cardiovasc. Med. 5, 571–579 (2008).
Gössl, M., Mödder, U. I., Atkinson, E. J., Lerman, A. & Khosla, S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J. Am. Coll. Cardiol. 52, 1314–1325 (2008).
Gössl, M. et al. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur. Heart J. 31, 2909–2914 (2010).
Egan, K. P., Kim, J. H., Mohler, E. R. 3rd & Pignolo, R. J. Role for circulating osteogenic precursor cells in aortic valvular disease. Arterioscler. Thromb. Vasc. Biol. 31, 2965–2971 (2011).
Eghbali-Fatourechi, G. Z. et al. Circulating osteoblast-lineage cells in humans. N. Engl. J. Med. 352, 1959–1966 (2005).
Shen, X. et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J. Orthop. Res. 27, 1298–1305 (2009).
Wan, C. et al. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl Acad. Sci. USA 105, 686–691 (2008).
Zelzer, E. & Olsen, B. R. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Curr. Top. Dev. Biol. 65, 169–187 (2005).
Demer, L. L. & Tintut, Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117, 2938–2948 (2008).
Shao, J. S., Cheng, S. L., Sadhu, J. & Towler, D. A. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension 55, 579–592 (2010).
Cheng, S. L. et al. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ. Res. 107, 271–282 (2010).
Shao, J. S., Cheng, S. L., Charlton-Kachigian, N., Loewy, A. P. & Towler, D. A. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J. Biol. Chem. 278, 50195–50202 (2003).
Shao, J. S. et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Invest. 115, 1210–1220 (2005).
Sebastian, E. M., Suva, L. J. & Friedman, P. A. Differential effects of intermittent PTH(1–34) and PTH(7–34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone 43, 1022–1030 (2008).
Huang, M. S. et al. Hyperlipidemia impairs osteoanabolic effects of PTH. J. Bone Miner. Res. 23, 1672–1679 (2008).
Huang, M. S. et al. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J. Biol. Chem. 282, 21237–21243 (2007).
Sage, A. P. et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J. Bone Miner. Res. 26, 1197–1206 (2011).
Murphy, W. A. Jr et al. The iceman: discovery and imaging. Radiology 226, 614–629 (2003).
Polonsky, T. S. et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 303, 1610–1616 (2010).
Rosenhek, R. et al. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur. Heart J. 25, 199–205 (2004).
Rosenhek, R. et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N. Engl. J. Med. 343, 611–617 (2000).
London, G. M. et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 18, 1731–1740 (2003).
Bos, D. et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler. Thromb. Vasc. Biol. 31, 2331–2337 (2011).
Stary, H. C. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 20, 1177–1178 (2000).
Burke, A. P., Kolodgie, F. D., Farb, A., Weber, D. & Virmani, R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 105, 297–303 (2002).
Ward, M. R., Pasterkamp, G., Yeung, A. C. & Borst, C. Arterial remodeling. Mechanisms and clinical implications. Circulation 102, 1186–1191 (2000).
Rajamannan, N. M. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler. Thromb. Vasc. Biol. 29, 162–168 (2009).
Rajamannan, N. M., Bonow, R. O. & Rahimtoola, S. H. Calcific aortic stenosis: an update. Nat. Clin. Pract. Cardiovasc. Med. 4, 254–262 (2007).
Greenwald, S. E. Ageing of the conduit arteries. J. Pathol. 211, 157–172 (2007).
Westerhof, N., Lankhaar, J. W. & Westerhof, B. E. The arterial Windkessel. Med. Biol. Eng. Comput. 47, 131–141 (2009).
O'Rourke, M. F. Arterial aging: pathophysiological principles. Vasc. Med. 12, 329–341 (2007).
Safar, M. E. & Boudier, H. S. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension 46, 205–209 (2005).
Virchow, R. & Chance, F. Cellular pathology, as based upon physiological and pathological histology. Twenty lectures delivered in the Pathological Institute of Berlin during the months of February, March and April, 1858 (R. M. De Witt, New York, 1860).
Virchow, R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI—Atheromatous affection of arteries. 1858. Nutr. Rev. 47, 23–25 (1989).
Mohler, E. R. 3rd et al. Bone formation and inflammation in cardiac valves. Circulation 103, 1522–1528 (2001).
Tyson, K. L. et al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler. Thromb. Vasc. Biol. 23, 489–494 (2003).
Hessle, L. et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl Acad. Sci. USA 99, 9445–9449 (2002).
Tanimura, A., McGregor, D. H. & Anderson, H. C. Calcification in atherosclerosis. I. Human studies. J. Exp. Pathol. 2, 261–273 (1986).
Tanimura, A., McGregor, D. H. & Anderson, H. C. Calcification in atherosclerosis. II. Animal studies. J. Exp. Pathol. 2, 275–297 (1986).
Boström, K. et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Invest. 91, 1800–1809 (1993).
Zhang, M. et al. Atorvastatin downregulates BMP-2 expression induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells. Circ. J. 72, 807–812 (2008).
Cola, C., Almeida, M., Li, D., Romeo, F. & Mehta, J. L. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem. Biophys. Res. Commun. 320, 424–427 (2004).
Tintut, Y. et al. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 105, 650–655 (2002).
Yao, Y. et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ. Res. 107, 485–494 (2010).
Towler, D. A. & Demer, L. L. Thematic series on the pathobiology of vascular calcification: an introduction. Circ. Res. 108, 1378–1380 (2011).
Stary, H. C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92, 1355–1374 (1995).
Chung, U. I., Kawaguchi, H., Takato, T. & Nakamura, K. Distinct osteogenic mechanisms of bones of distinct origins. J. Orthop. Sci. 9, 410–414 (2004).
Cohen, M. M. Jr. The new bone biology: pathologic, molecular, and clinical correlates. Am. J. Med. Genet. A 140, 2646–2706 (2006).
Abzhanov, A., Rodda, S. J., McMahon, A. P. & Tabin, C. J. Regulation of skeletogenic differentiation in cranial dermal bone. Development 134, 3133–3144 (2007).
Karsenty, G. Transcriptional control of skeletogenesis. Annu. Rev. Genomics Hum. Genet. 9, 183–196 (2008).
Aikawa, E. et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115, 377–386 (2007).
Steitz, S. A. et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 89, 1147–1154 (2001).
Speer, M. Y. et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 104, 733–741 (2009).
Speer, M. Y., Li, X., Hiremath, P. G. & Giachelli, C. M. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J. Cell. Biochem. 110, 935–947 (2010).
Demer, L. & Tintut, Y. The roles of lipid oxidation products and receptor activator of nuclear factor-kappaB signaling in atherosclerotic calcification. Circ. Res. 108, 1482–1493 (2011).
Richardson, J. A. et al. Oxysterol-induced osteoblastic differentiation of pluripotent mesenchymal cells is mediated through a PKC- and PKA-dependent pathway. J. Cell. Biochem. 100, 1131–1145 (2007).
Byon, C. H. et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 283, 15319–15327 (2008).
Narisawa, S. et al. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J. Bone Miner. Res. 22, 1700–1710 (2007).
Parhami, F. et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 17, 680–687 (1997).
Parhami, F. et al. Atherogenic high-fat diet reduces bone mineralization in mice. J. Bone Miner. Res. 16, 182–188 (2001).
Sage, A. P., Lu, J., Tintut, Y. & Demer, L. L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 79, 414–422 (2011).
Sage, A. P., Tintut, Y. & Demer, L. L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 7, 528–536 (2010).
Tintut, Y. et al. Multilineage potential of cells from the artery wall. Circulation 108, 2505–2510 (2003).
Collett, G. D. & Canfield, A. E. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ. Res. 96, 930–938 (2005).
Nelson, R. G. et al. Lower-extremity amputations in NIDDM. 12-yr follow-up study in Pima Indians. Diabetes Care 11, 8–16 (1988).
Leskinen, Y., Salenius, J. P., Lehtimäki, T., Huhtala, H. & Saha, H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am. J. Kidney Dis. 40, 472–479 (2002).
Towler, D. A. Vascular calcification: A perspective on an imminent disease epidemic. IBMS BoneKEy 5, 41–58 (2008).
Robbins, S. L., Cotran, R. S. & Kumar, V. Pathologic Basis of Disease (Saunders, Philadelphia, 1984).
O'Rourke, M. F. & Hashimoto, J. Mechanical factors in arterial aging: a clinical perspective. J. Am. Coll. Cardiol. 50, 1–13 (2007).
Al-Aly, Z. et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-α -dependent signals in diabetic Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 27, 2589–2596 (2007).
Qiao, J. H., Mertens, R. B., Fishbein, M. C. & Geller, S. A. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum. Pathol. 34, 402–407 (2003).
Satokata, I. et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 24, 391–395 (2000).
Towler, D. A., Bidder, M., Latifi, T., Coleman, T. & Semenkovich, C. F. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J. Biol. Chem. 273, 30427–30434 (1998).
Canfield, A. E. et al. Role of pericytes in vascular calcification: a review. Z. Kardiol. 89 (Suppl. 2), 20–27 (2000).
Zhang, L. et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation 108, 472–478 (2003).
Csiszar, A. et al. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-α, H2O2, and high intravascular pressure. Circulation 111, 2364–2372 (2005).
Rawadi, G., Vayssière, B., Dunn, F., Baron, R. & Roman-Roman, S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 18, 1842–1853 (2003).
Cheng, S. L., Shao, J. S., Charlton-Kachigian, N., Loewy, A. P. & Towler, D. A. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 278, 45969–45977 (2003).
Shao, J. S., Cai, J. & Towler, D. A. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 26, 1423–1430 (2006).
Cheng, S. L., Shao, J. S., Cai, J., Sierra, O. L. & Towler, D. A. Msx2 exerts bone anabolism via canonical Wnt signaling. J. Biol. Chem. 283, 20505–20522 (2008).
Boström, K. I., Rajamannan, N. M. & Towler, D. A. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ. Res. 109, 564–577 (2011).
Orrico, C. et al. Dysfunctional vasa vasorum in diabetic peripheral artery obstructive disease with critical lower limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 40, 365–374 (2010).
Hayden, M. R. & Tyagi, S. C. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc. Diabetol. 3, 1 (2004).
Buján, J. et al. Modifications induced by atherogenic diet in the capacity of the arterial wall in rats to respond to surgical insult. Atherosclerosis 122, 141–152 (1996).
Miller, J. D. et al. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 52, 843–850 (2008).
Caira, F. C. et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J. Am. Coll. Cardiol. 47, 1707–1712 (2006).
Rajamannan, N. M. The role of Lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the ApoE−/−/Lrp5−/− mice. J. Cell. Biochem. 112, 2987–2991 (2011).
Hellberg, C., Ostman, A. & Heldin, C. H. PDGF and vessel maturation. Recent Results Cancer Res. 180, 103–114 (2010).
Fadini, G. P. et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ. Res. 108, 1112–1121 (2011).
Pignolo, R. J. & Shore, E. M. Circulating osteogenic precursor cells. Crit. Rev. Eukaryot. Gene Expr. 20, 171–180 (2010).
Hruska, K. A., Choi, E. T., Memon, I., Davis, T. K. & Mathew, S. Cardiovascular risk in chronic kidney disease (CKD): the CKD-mineral bone disorder (CKD–MBD). Pediatr. Nephrol. 25, 769–778 (2010).
Moe, S. M., Drüeke, T., Lameire, N. & Eknoyan, G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv. Chronic Kidney Dis. 14, 3–12 (2007).
Foley, R. N. & Collins, A. J. End-stage renal disease in the United States: an update from the United States Renal Data System. J. Am. Soc. Nephrol. 18, 2644–2648 (2007).
London, G. M. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J. Am. Soc. Nephrol. 14 (Suppl. 4), S305–S309 (2003).
Ishimura, E. et al. Different risk factors for vascular calcification in end-stage renal disease between diabetics and nondiabetics: the respective importance of glycemic and phosphate control. Kidney Blood Press. Res. 31, 10–15 (2008).
Mizobuchi, M., Towler, D. & Slatopolsky, E. Vascular calcification: the killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 20, 1453–1464 (2009).
Moe, S. M. et al. The pathophysiology of early stage chronic kidney disease-mineral bone disorder (CKD–MBD) and response to phosphate binders in the rat. J. Bone Miner. Res. 26, 2672–2681 (2011).
Foley, R. N., Collins, A. J., Herzog, C. A., Ishani, A. & Kalra, P. A. Serum phosphate and left ventricular hypertrophy in young adults: the coronary artery risk development in young adults study. Kidney Blood Press. Res. 32, 37–44 (2009).
Foley, R. N., Collins, A. J., Herzog, C. A., Ishani, A. & Kalra, P. A. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J. Am. Soc. Nephrol. 20, 397–404 (2009).
Reynolds, J. L. et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15, 2857–2867 (2004).
Reynolds, J. L. et al. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J. Am. Soc. Nephrol. 16, 2920–2930 (2005).
Jahnen-Dechent, W., Heiss, A., Schäfer, C. & Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 108, 1494–1509 (2011).
Ciaccio, M. et al. Changes in serum fetuin-A and inflammatory markers levels in end-stage renal disease (ESRD): effect of a single session haemodialysis. Clin. Chem. Lab. Med. 46, 212–214 (2008).
Villa-Bellosta, R., Levi, M. & Sorribas, V. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-α, and Pi. Pflugers Arch. 458, 1151–1161 (2009).
Li, X., Yang, H. Y. & Giachelli, C. M. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 199, 271–277 (2008).
Shroff, R. C. et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118, 1748–1757 (2008).
Nadra, I. et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ. Res. 96, 1248–1256 (2005).
Boström, K. Proinflammatory vascular calcification. Circ. Res. 96, 1219–1220 (2005).
Koleganova, N. et al. Arterial calcification in patients with chronic kidney disease. Nephrol. Dial. Transplant. 24, 2488–2496 (2009).
Fellström, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407 (2009).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Block, G. A., Raggi, P., Bellasi, A., Kooienga, L. & Spiegel, D. M. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 71, 438–441 (2007).
Moe, S. M. & Chertow, G. M. The case against calcium-based phosphate binders. Clin. J. Am. Soc. Nephrol. 1, 697–703 (2006).
Raggi, P., Vukicevic, S., Moysés, R. M., Wesseling, K. & Spiegel, D. M. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin. J. Am. Soc. Nephrol. 5 (Suppl. 1), S31–S40 (2010).
Raggi, P. et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J. Bone Miner. Res. 20, 764–772 (2005).
London, G. M. et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J. Am. Soc. Nephrol. 15, 1943–1951 (2004).
Demer, L. & Tintut, Y. The bone–vascular axis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 19, 349–353 (2010).
Findlay, D. M. & Martin, T. J. Receptors of calciotropic hormones. Horm. Metab. Res. 29, 128–134 (1997).
Chambliss, K. L. et al. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res. 87, E44–E52 (2000).
Ferreira, J. A., Pompei, L. M., Fernandes, C. E., Azevedo, L. H. & Peixoto, S. Breast arterial calcification is a predictive factor of cardiovascular disease in Brazilian postmenopausal women. Climacteric 12, 439–444 (2009).
Barrett-Connor, E. & Laughlin, G. A. Hormone therapy and coronary artery calcification in asymptomatic postmenopausal women: the Rancho Bernardo Study. Menopause 12, 40–48 (2005).
Rossouw, J. E. et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297, 1465–1477 (2007).
Van Den Bosch, M. A. et al. The RATIO study: oral contraceptives and the risk of peripheral arterial disease in young women. J. Thromb. Haemost. 1, 439–444 (2003).
Bukoski, R. D. & Kremer, D. Calcium-regulating hormones in hypertension: vascular actions. Am. J. Clin. Nutr. 54 (Suppl.), 220S–226S (1991).
Holick, M. F. Sunlight and vitamin D: both good for cardiovascular health. J. Gen. Intern. Med. 17, 733–735 (2002).
Brewer, L. C., Michos, E. D. & Reis, J. P. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr. Drug Targets 12, 54–60 (2011).
Oh, J. et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 120, 687–698 (2009).
Neuhouser, M. L. et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Arch. Intern. Med. 169, 294–304 (2009).
Querfeld, U. & Mak, R. H. Vitamin D deficiency and toxicity in chronic kidney disease: in search of the therapeutic window. Pediatr. Nephrol. 25, 2413–2430 (2010).
Price, P. A., Williamson, M. K., Nguyen, T. M. & Than, T. N. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J. Biol. Chem. 279, 1594–1600 (2004).
Idelevich, A., Rais, Y. & Monsonego-Ornan, E. Bone Gla protein increases HIF-1α-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler. Thromb. Vasc. Biol. 31, e55–e71 (2011).
Alam, M. U. et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc. Res. 81, 260–268 (2009).
Henley, C. et al. 1,25-Dihydroxyvitamin = D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol. Dial. Transplant. 20, 1370–1377 (2005).
Jono, S., Nishizawa, Y., Shioi, A. & Morii, H. Parathyroid hormone-related peptide as a local regulator of vascular calcification. Its inhibitory action on in vitro calcification by bovine vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 17, 1135–1142 (1997).
Fiaschi-Taesch, N. et al. Mutant parathyroid hormone-related protein, devoid of the nuclear localization signal, markedly inhibits arterial smooth muscle cell cycle and neointima formation by coordinate up-regulation of p15Ink4b and p27kip1. Endocrinology 150, 1429–1439 (2009).
Fiaschi-Taesch, N., Takane, K. K., Masters, S., Lopez-Talavera, J. C. & Stewart, A. F. Parathyroid-hormone-related protein as a regulator of pRb and the cell cycle in arterial smooth muscle. Circulation 110, 177–185 (2004).
Qian, J. et al. Midgestational lethality in mice lacking the parathyroid hormone (PTH)/PTH-related peptide receptor is associated with abrupt cardiomyocyte death. Endocrinology 144, 1053–1061 (2003).
Nyby, M. D. et al. Desensitization of vascular tissue to parathyroid hormone and parathyroid hormone-related protein. Endocrinology 136, 2497–2504 (1995).
Schipani, E., Kruse, K. & Jüppner, H. A constitutively active mutant PTH–PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science 268, 98–100 (1995).
Langub, M. C. et al. Administration of PTH-(7–84) antagonizes the effects of PTH-(1–84) on bone in rats with moderate renal failure. Endocrinology 144, 1135–1138 (2003).
Walker, M. D. et al. Carotid vascular abnormalities in primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 94, 3849–3856 (2009).
Maeda, S. et al. Targeted overexpression of parathyroid hormone-related protein (PTHrP) to vascular smooth muscle in transgenic mice lowers blood pressure and alters vascular contractility. Endocrinology 140, 1815–1825 (1999).
Song, G. J. et al. EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells. J. Mol. Cell. Cardiol. 49, 1012–1021 (2010).
Suttamanatwong, S., Franceschi, R. T., Carlson, A. E. & Gopalakrishnan, R. Regulation of matrix Gla protein by parathyroid hormone in MC3T3-E1 osteoblast-like cells involves protein kinase A and extracellular signal-regulated kinase pathways. J. Cell. Biochem. 102, 496–505 (2007).
Gopalakrishnan, R., Suttamanatwong, S., Carlson, A. E. & Franceschi, R. T. Role of matrix Gla protein in parathyroid hormone inhibition of osteoblast mineralization. Cells Tissues Organs 181, 166–175 (2005).
Brenneise, C. V. & Squier, C. A. Blood flow in maxilla and mandible of normal and atherosclerotic rhesus monkeys. J. Oral Pathol. 14, 800–808 (1985).
Shao, J. S. et al. Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 1821–1833 (2011).
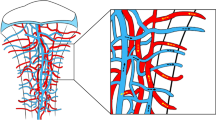
McCarthy, I. The physiology of bone blood flow: a review. J. Bone Joint Surg. Am. 88 (Suppl. 3), 4–9 (2006).
Bridgeman, G. & Brookes, M. Blood supply to the human femoral diaphysis in youth and senescence. J. Anat. 188, 611–621 (1996).
Brinker, M. R., Lippton, H. L., Cook, S. D. & Hyman, A. L. Pharmacological regulation of the circulation of bone. J. Bone Joint Surg. Am. 72, 964–975 (1990).
Prisby, R. D. et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J. Bone Miner. Res. 22, 1280–1288 (2007).
Prisby, R. et al. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone forming sites. J. Bone Miner. Res. 26, 2583–2596 (2011).
Bouletreau, P. J. et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast. Reconstr. Surg. 109, 2384–2397 (2002).
Zhao, C. et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 4, 111–121 (2006).
Bochenek, M. L., Dickinson, S., Astin, J. W., Adams, R. H. & Nobes, C. D. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J. Cell Sci. 123, 1235–1246 (2010).
Kanazawa, I. et al. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 8, 51 (2007).
Armour, K. E. et al. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142, 760–766 (2001).
Collin-Osdoby, P. et al. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J. Biol. Chem. 276, 20659–20672 (2001).
Dominguez, J. M. 2nd, Prisby, R. D., Muller-Delp, J. M., Allen, M. R. & Delp, M. D. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone 46, 813–819 (2010).
Bianco, P., Sacchetti, B. & Riminucci, M. Osteoprogenitors and the hematopoietic microenvironment. Best Pract. Res. Clin. Haematol. 24, 37–47 (2011).
Brunner, S. et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp. Hematol. 36, 1157–1166 (2008).
Fukumoto, S. & Martin, T. J. Bone as an endocrine organ. Trends Endocrinol. Metab. 20, 230–236 (2009).
Gutierrez, O. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 (2005).
Bergwitz, C. & Jüppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 61, 91–104 (2010).
Sitara, D. et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am. J. Pathol. 169, 2161–2170 (2006).
Fukumoto, S. & Yamashita, T. FGF23 is a hormone-regulating phosphate metabolism–unique biological characteristics of FGF23. Bone 40, 1190–1195 (2007).
Stubbs, J. R. et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 18, 2116–2124 (2007).
Isakova, T. et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J. Am. Soc. Nephrol. 19, 615–623 (2008).
Shimizu, H. et al. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-kB. Am. J. Nephrol. 33, 319–324 (2011).
Farrow, E. G. & White, K. E. Recent advances in renal phosphate handling. Nat. Rev. Nephrol. 6, 207–217 (2010).
Canalejo, R. et al. FGF23 fails to inhibit uremic parathyroid glands. J. Am. Soc. Nephrol. 21, 1125–1135 (2010).
Adijiang, A. & Niwa, T. An oral sorbent, AST-120, increases Klotho expression and inhibits cell senescence in the kidney of uremic rats. Am. J. Nephrol. 31, 160–164 (2010).
Hu, M. C. et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 22, 124–136 (2011).
Saito, Y. et al. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun. 248, 324–329 (1998).
Wacker, M. et al. Dentin matrix protein 1 null mice, a model of human autosomal recessive hyphosphatemic rickets, exhibit decreases in cardiac, skeletal, and vascular smooth muscle function [abstract]. J. Bone Miner. Res. 25 (Suppl. 1), a1157 (2010).
Jono, S., Peinado, C. & Giachelli, C. M. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J. Biol. Chem. 275, 20197–20203 (2000).
Christensen, B. et al. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J. Biol. Chem. 282, 19463–19472 (2007).
Scatena, M., Liaw, L. & Giachelli, C. M. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 27, 2302–2309 (2007).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007).
Whitehead, J. P., Richards, A. A., Hickman, I. J., Macdonald, G. A. & Prins, J. B. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 8, 264–280 (2006).
Son, B. K. et al. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-α on vascular smooth muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5'-monophosphate-activated protein kinase. Endocrinology 149, 1646–1653 (2008).
Luo, X. H. et al. Development of arterial calcification in adiponectin-deficient mice: adiponectin regulates arterial calcification. J. Bone Miner. Res. 24, 1461–1468 (2009).
Lomonte, C. et al. Serum parathyroid hormone and phosphate influence the levels of circulating CD34+ cells in uremia. J. Nephrol. 23, 693–698 (2010).
Faul, C. et al. FGF23 induces left ventricular hypertrophy.. J. Clin. Invest. 121, 4393–4408 (2011).
Yu, N. et al. Increased mortality and morbidity in mild primary hyperparathyroid patients. The Parathyroid Epidemiology and Audit Research Study (PEARS). Clin. Endocrinol. (Oxf.) 73, 30–34 (2010).
Rhee, Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011).
Sage, A. P. et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J. Bone Miner. Res. 26, 1197–1206 (2011).
López, I. et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 80, 475–482 (2011).
Kosch, M. et al. Impaired flow-mediated vasodilation of the brachial artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc. Res. 47, 813–818 (2000).
Teng, M. et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N. Engl. J. Med. 349, 446–456 (2003).
Golestani, R. et al. Abdominal aortic calcification detected by dual X-ray absorptiometry: a strong predictor for cardiovascular events. Ann. Med. 42, 539–545 (2010).
Sennerby, U. et al. Cardiovascular diseases and risk of hip fracture. JAMA 302, 1666–1673 (2009).
von Mühlen, D., Allison, M., Jassal, S. K. & Barrett-Connor, E. Peripheral arterial disease and osteoporosis in older adults: the Rancho Bernardo Study. Osteoporos. Int. 20, 2071–2078 (2009).
Bagger, Y. Z. et al. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos. Int. 18, 505–512 (2007).
Elmariah, S. et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 56, 1752–1759 (2010).
Bolland, M. J. et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 336, 262–266 (2008).
Bolland, M. J., Grey, A., Avenell, A., Gamble, G. D. & Reid, I. R. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 342, d2040 (2011).
Yoshimura, T. et al. Impaired peripheral circulation in lower-leg arteries caused by higher arterial stiffness and greater vascular resistance associates with nephropathy in type 2 diabetic patients with normal ankle–brachial indices. Diabetes Res. Clin. Pract. 80, 416–423 (2008).
Johnson, K. A., Polewski, M. & Terkeltaub, R. A. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ. Res. 102, 529–537 (2008).
Faverman, L., Mikhaylova, L., Malmquist, J. & Nurminskaya, M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 582, 1552–1557 (2008).
Towler, D. A. Calciotropic hormones and arterial physiology: “D”-lightful insights. J. Am. Soc. Nephrol. 18, 369–373 (2007).
Shroff, R. et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J. Am. Soc. Nephrol. 19, 1239–1246 (2008).
Behnke, B. J. et al. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J. Physiol. 575, 617–626 (2006).
Kronenberg, H. M. PTH regulates the hematopoietic stem cell niche in bone. Adv. Exp. Med. Biol. 602, 57–60 (2007).
Jilka, R. L. et al. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 9, 851–867 (2010).
Acknowledgements
D. A. Towler was supported by NIH grants HL88651, HL69299, and HL81138, and the Barnes-Jewish Hospital Foundation.
Author information
Authors and Affiliations
Contributions
D. A. Towler researched the data for the article and wrote the article. Both authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Thompson, B., Towler, D. Arterial calcification and bone physiology: role of the bone–vascular axis. Nat Rev Endocrinol 8, 529–543 (2012). https://doi.org/10.1038/nrendo.2012.36
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.36
This article is cited by
-
Impact of Cinacalcet and Etelcalcetide on Bone Mineral and Cardiovascular Disease in Dialysis Patients
Current Osteoporosis Reports (2023)
-
Black phosphorous nanomaterials as a new paradigm for postoperative tumor treatment regimens
Journal of Nanobiotechnology (2022)
-
Silencing of STE20-type kinase STK25 in human aortic endothelial and smooth muscle cells is atheroprotective
Communications Biology (2022)
-
Skeletal and extraskeletal disorders of biomineralization
Nature Reviews Endocrinology (2022)
-
MiR-133a is a potential target for arterial calcification in patients with end-stage renal disease
International Urology and Nephrology (2022)