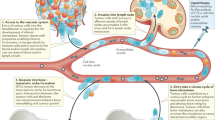
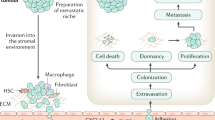
Abstract
The skeleton is one of the most common sites for metastatic cancer, and tumors arising from the breast or prostate possess an increased propensity to spread to this site. The growth of disseminated tumor cells in the skeleton requires tumor cells to inhabit the bone marrow, from which they stimulate local bone cell activity. Crosstalk between tumor cells and resident bone and bone marrow cells disrupts normal bone homeostasis, which leads to tumor growth in bone. The metastatic tumor cells have the ability to elicit responses that stimulate bone resorption, bone formation or both. The net result of these activities is profound skeletal destruction that can have dire consequences for patients. The molecular mechanisms that underlie these painful and often incurable consequences of tumor metastasis to bone are beginning to be recognized, and they represent promising new molecular targets for therapy.
Key Points
-
Bone is a common site of metastasis for many tumors, such as breast, lung and prostate cancer
-
A continuum of bone metastasis exists that extends from primarily osteolytic lesions with limited osteoblast activity to bone metastases that are predominantly osteoblastic, with limited osteoclast activity
-
The complex interactions between circulating tumor cells, circulating host cells, platelets and cell-derived factors are critical for tumor establishment at metastatic sites
-
Tumor activation of bone resorption is complex and involves both receptor activator of nuclear factor κB ligand (RANKL)-dependent and RANKL-independent mechanisms
-
Targeting bone resorption with bisphosphonates reduces osteolytic bone resorption and improves disease-free survival
-
All steps in the metastatic process and the interaction with host cells are valid therapeutic targets for the treatment of bone metastasis and tumor progression
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
DeNardo, D. G., Johansson, M. & Coussens, L. M. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 27, 11–18 (2008).
Amano, H. et al. Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase-9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer Sci. 100, 2318–2324 (2009).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
de Visser, K. E., Korets, L. V. & Coussens, L. M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7, 411–423 (2005).
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Poste, G. & Fidler, I. J. The pathogenesis of cancer metastasis. Nature 283, 139–146 (1980).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Roodman, G. D. Mechanisms of bone metastasis. N. Engl. J. Med. 350, 1655–1664 (2004).
Suva, L. J., Griffin, R. J. & Makhoul, I. Mechanisms of bone metastases of breast cancer. Endocr. Relat. Cancer 16, 703–713 (2009).
Coleman, R. E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243S–6249S (2006).
León, X., Quer, M., Orús, C., del Prado Venegas, M. & López, M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck 22, 680–686 (2000).
Patten, R. M., Shuman, W. P. & Teefey, S. Metastases from malignant melanoma to the axial skeleton: a CT study of frequency and appearance. AJR Am. J. Roentgenol. 155, 109–112 (1990).
Bendre, M., Gaddy, D., Nicholas, R. W. & Suva, L. J. Breast cancer metastasis to bone: it is not all about PTHrP. Clin. Orthop. Relat. Res. 415 (Suppl.), S39–S45 (2003).
Casimiro, S., Guise, T. A. & Chirgwin, J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell Endocrinol. 310, 71–81 (2009).
Guise, T. A. et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 12, 6213S–6216S (2006).
Coleman, R. E. Skeletal complications of malignancy. Cancer 80 (Suppl.), 1588–1594 (1997).
Coleman, R. et al. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat. Rev. 34, 629–639 (2008).
Demers, L. M. et al. Biochemical markers of bone turnover in patients with metastatic bone disease. Clin. Chem. 41, 1489–1494 (1995).
Martin, T. J. Manipulating the environment of cancer cells in bone: a novel therapeutic approach. J. Clin. Invest. 110, 1399–1401 (2002).
Boyde, A., Maconnachie, E., Reid, S. A., Delling, G. & Mundy, G. R. Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scan. Electron. Microsc. 4, 1537–1554 (1986).
Mundy, G. R. & Martin, T. J. Physiology and Pharmacology of Bone 641–671 (Springer-Verlag, Berlin, 1993).
Stewart, A. F. et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J. Clin. Endocrinol. Metab. 55, 219–227 (1982).
Charhon, S. A. et al. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer 51, 918–924 (1983).
Roudier, M. P. et al. Histopathological assessment of prostate cancer bone osteoblastic metastases. J. Urol. 180, 1154–1160 (2008).
Coleman, R. E. Conclusion: Bone markers in metastatic bone disease. Cancer Treat. Rev. 32 (Suppl. 1), 27–28 (2006).
Fili, S., Karalaki, M. & Schaller, B. Mechanism of bone metastasis: the role of osteoprotegerin and of the host-tissue microenvironment-related survival factors. Cancer Lett. 283, 10–19 (2009).
Percival, R. C. et al. Biochemical and histological evidence that carcinoma of the prostate is associated with increased bone resorption. Eur. J. Surg. Oncol. 13, 41–49 (1987).
Pelger, R. C., Hamdy, N. A., Zwinderman, A. H., Lycklama à Nijeholt, A. A. & Papapoulos, S. E. Effects of the bisphosphonate olpadronate in patients with carcinoma of the prostate metastatic to the skeleton. Bone 22, 403–408 (1998).
Garnero, P. et al. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br. J. Cancer 82, 858–864 (2000).
Yi, B., Williams, P. J., Niewolna, M., Wang, Y. & Yoneda, T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 62, 917–923 (2002).
Yin, J. J. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl Acad. Sci. USA 100, 10954–10959 (2003).
Thalmann, G. N. et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate 44, 91–103 (2000).
LeRoy, B. E. et al. New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate 66, 1213–1222 (2006).
Power, C. A. et al. A novel model of bone-metastatic prostate cancer in immunocompetent mice. Prostate 69, 1613–1623 (2009).
Rodan, G. A. & Martin, T. J. Therapeutic approaches to bone diseases. Science 289, 1508–1514 (2000).
Roodman, G. D. Pathogenesis of myeloma bone disease. Blood Cells Mol. Dis. 32, 290–292 (2004).
Pennisi, A. et al. Inhibitor of DASH proteases affects expression of adhesion molecules in osteoclasts and reduces myeloma growth and bone disease. Br. J. Haematol. 145, 775–787 (2009).
Tian, E. et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349, 2483–2494 (2003).
Jia, D. et al. Repression of multiple myeloma growth and preservation of bone with combined radiotherapy and anti-angiogenic agent. Radiat. Res. 173, 809–817 (2010).
Stokkel, M. P., Linthorst, M. F., Borm, J. J., Taminiau, A. H. & Pauwels, E. K. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. J. Cancer Res. Clin. Oncol. 128, 393–399 (2002).
Bussard, K. M., Gay, C. V. & Mastro, A. M. The bone microenvironment in metastasis: what is special about bone? Cancer Metastasis Rev. 27, 41–55 (2008).
Fidler, I. J., Gersten, D. M. & Hart, I. R. The biology of cancer invasion and metastasis. Adv. Cancer Res. 28, 149–250 (1978).
Suva, L. J. Adjuvant bisphosphonates in breast cancer: the ABCSG-12 study. Curr. Osteoporos. Rep. 8, 57–59 (2010).
Boucharaba, A. et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Invest. 114, 1714–1725 (2004).
Bendre, M. S. et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 65, 11001–11009 (2005).
Jain, S. et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc. Natl Acad. Sci. USA 104, 9024–9028 (2007).
Suva, L. J. et al. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am. J. Pathol. 172, 430–439 (2008).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Chavez-Macgregor, M. et al. Angiogenesis in the bone marrow of patients with breast cancer. Clin. Cancer Res. 11, 5396–5400 (2005).
Suva, L. J., Gaddy, D., Perrien, D. S., Thomas, R. L. & Findlay, D. M. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr. Osteoporos. Rep. 3, 46–51 (2005).
Sanderson, R. D., Yang, Y., Suva, L. J. & Kelly, T. Heparan sulfate proteoglycans and heparanase--partners in osteolytic tumor growth and metastasis. Matrix Biol. 23, 341–352 (2004).
Carcel-Trullols, J. et al. Characterization of the glycosylation profile of the human breast cancer cell line, MDA-231, and a bone colonizing variant. Int. J. Oncol. 28, 1173–1183 (2006).
Yang, Y. et al. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood 110, 2041–2048 (2007).
Vallet, S. et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc. Natl Acad. Sci. USA 107, 5124–5129 (2010).
Scheel, C., Onder, T., Karnoub, A. & Weinberg, R. A. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 67, 11476–11479 (2007).
Li, F., Tiede, B., Massagué, J. & Kang, Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 17, 3–14 (2007).
Talmadge, J. E. Clonal selection of metastasis within the life history of a tumor. Cancer Res. 67, 11471–11475 (2007).
Kelly, P. N., Dakic, A., Adams, J. M., Nutt, S. L. & Strasser, A. Tumor growth need not be driven by rare cancer stem cells. Science 317, 337 (2007).
Shi, Y. & Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Roberts, A. B. & Sporn, M. B. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors 8, 1–9 (1993).
Fournier, P. G., Chirgwin, J. M. & Guise, T. A. New insights into the role of T cells in the vicious cycle of bone metastases. Curr. Opin. Rheumatol. 18, 396–404 (2006).
Arya, M., Ahmed, H., Silhi, N., Williamson, M. & Patel, H. R. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumour Biol. 28, 123–131 (2007).
Kang, H. et al. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 7, R402–R410 (2005).
Massagué, J. & Gomis, R. R. The logic of TGFbeta signaling. FEBS Lett. 580, 2811–2820 (2006).
Dallas, S. L., Rosser, J. L., Mundy, G. R. & Bonewald, L. F. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J. Biol. Chem. 277, 21352–21360 (2002).
Mohammad, K. S. et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One 4, e5275 (2009).
Suva, L. J. et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science 237, 893–896 (1987).
Burtis, W. J. et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N. Engl. J. Med. 322, 1106–1112 (1990).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Roodman, G. D. & Dougall, W. C. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat. Rev. 34, 92–101 (2008).
Southby, J. et al. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res. 50, 7710–7716 (1990).
Powell, G. J. et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 51, 3059–3061 (1991).
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).
Henderson, M. A. et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 66, 2250–2256 (2006).
Sterling, J. A., Edwards, J. R., Martin, T. J. & Mundy, G. R. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone doi: 10.1016/j.bone.2010.07.015.
Lu, Y. et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 67, 3646–3653 (2007).
Lau, Y. S. et al. RANKL-dependent and RANKL-independent mechanisms of macrophage-osteoclast differentiation in breast cancer. Breast Cancer Res. Treat. 105, 7–16 (2007).
Weitzmann, M. N., Cenci, S., Rifas, L., Brown, C. & Pacifici, R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood 96, 1873–1878 (2000).
Kudo, O. et al. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32, 1–7 (2003).
Quinn, J. M. et al. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J. Bone Miner. Res. 16, 1787–1794 (2001).
Kelly, T. et al. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res. 65, 5778–5784 (2005).
Kelly, T., Suva, L. J., Nicks, K. M., MacLeod, V. & Sanderson, R. D. Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis. J. Bone Miner. Res. 25, 1295–1304 (2010).
Fizazi, K. et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 27, 1564–1571 (2009).
Vallet, S., Smith, M. R. & Raje, N. Novel bone-targeted strategies in oncology. Clin. Cancer Res. 16, 4084–4093 (2010).
Body, J. J. et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin. Cancer Res. 12, 1221–1228 (2006).
Lipton, A. et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 25, 4431–4437 (2007).
Lipton, A. et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin. Cancer Res. 14, 6690–6696 (2008).
Vij, R. et al. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. Am. J. Hematol. 84, 650–656 (2009).
McClung, M. R. et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 354, 821–831 (2006).
Nambi, P., Wu, H. L., Lipshutz, D. & Prabhakar, U. Identification and characterization of endothelin receptors on rat osteoblastic osteosarcoma cells: down-regulation by 1,25-dihydroxy-vitamin D3. Mol. Pharmacol. 47, 266–271 (1995).
Guise, T. A., Yin, J. J. & Mohammad, K. S. Role of endothelin-1 in osteoblastic bone metastases. Cancer 97 (Suppl. 3), 779–784 (2003).
Van Sant, C. et al. Endothelin signaling in osteoblasts: global genome view and implication of the calcineurin/NFAT pathway. Mol. Cancer Ther. 6, 253–261 (2007).
Mansson, P. E., Adams, P., Kan, M. & McKeehan, W. L. Heparin-binding growth factor gene expression and receptor characteristics in normal rat prostate and two transplantable rat prostate tumors. Cancer Res. 49, 2485–2494 (1989).
Matuo, Y. et al. Heparin binding affinity of rat prostatic growth factor in normal and cancerous prostates: partial purification and characterization of rat prostatic growth factor in the Dunning tumor. Cancer Res. 47, 188–192 (1987).
Morrissey, C., Brown, L. G., Pitts, T. E., Vessella, R. L. & Corey, E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia 12, 192–205 (2010).
Bailey, J. M., Singh, P. K. & Hollingsworth, M. A. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell. Biochem. 102, 829–839 (2007).
Harris, S. E. et al. Expression of bone morphogenetic protein messenger RNAs by normal rat and human prostate and prostate cancer cells. Prostate 24, 204–211 (1994).
Kim, I. Y. et al. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer Res. 60, 2840–2844 (2000).
Brubaker, K. D., Corey, E., Brown, L. G. & Vessella, R. L. Bone morphogenetic protein signaling in prostate cancer cell lines. J. Cell. Biochem. 91, 151–160 (2004).
Feeley, B. T. et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J. Bone Miner. Res. 20, 2189–2199 (2005).
Ye, L., Lewis-Russell, J. M., Kynaston, H. & Jiang, W. G. Endogenous bone morphogenetic protein-7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein antagonists. J. Urol. 178, 1086–1091 (2007).
Schwertfeger, K. L. Fibroblast growth factors in development and cancer: insights from the mammary and prostate glands. Curr. Drug Targets 10, 632–644 (2009).
Kwabi-Addo, B., Ozen, M. & Ittmann, M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr. Relat. Cancer 11, 709–724 (2004).
Memarzadeh, S. et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell 12, 572–585 (2007).
Acevedo, V. D. et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell 12, 559–571 (2007).
Mayahara, H. et al. In vivo stimulation of endosteal bone formation by basic fibroblast growth factor in rats. Growth Factors 9, 73–80 (1993).
Izbicka, E. et al. Human amniotic tumor that induces new bone formation in vivo produces growth-regulatory activity in vitro for osteoblasts identified as an extended form of basic fibroblast growth factor. Cancer Res. 56, 633–636 (1996).
Dunstan, C. R. et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J. Bone Miner. Res. 14, 953–959 (1999).
Kodama, N. et al. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone 44, 699–707 (2009).
Bennett, C. N. et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl Acad. Sci. USA 102, 3324–3329 (2005).
Hall, C. L. & Keller, E. T. The role of Wnts in bone metastases. Cancer Metastasis Rev. 25, 551–558 (2006).
Hall, C. L., Bafico, A., Dai, J., Aaronson, S. A. & Keller, E. T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 65, 7554–7560 (2005).
Clines, G. A. et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol. Endocrinol. 21, 486–498 (2007).
Gilbey, A. M., Burnett, D., Coleman, R. E. & Holen, I. The detection of circulating breast cancer cells in blood. J. Clin. Pathol. 57, 903–911 (2004).
Vessella, R. L. & Corey, E. Targeting factors involved in bone remodeling as treatment strategies in prostate cancer bone metastasis. Clin. Cancer Res. 12, 6285s–6290s (2006).
Coleman, R. E. Adjuvant bisphosphonates in breast cancer: are we witnessing the emergence of a new therapeutic strategy? Eur. J. Cancer 45, 1909–1915 (2009).
Gnant, M. et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 360, 679–691 (2009).
Stopeck, A. T. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. doi: 10.1200/JCO.2010.29.7101.
Gonzalez-Angulo, A. M., Morales-Vasquez, F. & Hortobagyi, G. N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 608, 1–22 (2007).
Weigelt, B., Peterse, J. L. & van 't Veer, L. J. Breast cancer metastasis: markers and models. Nat. Rev. Cancer 5, 591–602 (2005).
Vaage, J. Metastasizing potentials of mouse mammary tumors and their metastases. Int. J. Cancer 41, 855–858 (1988).
Hill, R. P., Chambers, A. F., Ling, V. & Harris, J. F. Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells. Science 224, 998–1001 (1984).
Acknowledgements
The authors dedicate this manuscript to the memory of Dr. Gregory R. Mundy. A fellow ex-patriot Australian, Greg's boundless energy, determination and drive virtually single-handedly motivated all of us to acknowledge and appreciate the intricacies of the bone–tumor microenvironment. His presence, unique perspective and intellect are sorely missed. Work into the mechanisms of cancer progression and bone metastases described in this Review was supported by NIH 5R01CA107160 (R. J. Griffin) and the Carl L. Nelson endowed Chair in Orthopedic Creativity (L. J. Suva).
Author information
Authors and Affiliations
Contributions
L. J. Suva and C. Washam researched the data for the article. L. J. Suva and R. W. Nicholas provided substantial contributions to discussions of the content. L. J. Suva and R. J. Griffin contributed equally to writing the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Suva, L., Washam, C., Nicholas, R. et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol 7, 208–218 (2011). https://doi.org/10.1038/nrendo.2010.227
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2010.227
This article is cited by
-
The progressive trend of modeling and drug screening systems of breast cancer bone metastasis
Journal of Biological Engineering (2024)
-
Aussagekraft von Prokollagen Typ I aminoterminales Propeptid (P1NP) als Knochenaufbaumarker
Zentralblatt für Arbeitsmedizin, Arbeitsschutz und Ergonomie (2024)
-
Osteoblastic bone reaction in non-small cell lung cancer harboring epidermal growth factor receptor mutation treated with osimertinib
BMC Cancer (2023)
-
New insights into the correlations between circulating tumor cells and target organ metastasis
Signal Transduction and Targeted Therapy (2023)
-
EHD1-dependent traffic of IGF-1 receptor to the cell surface is essential for Ewing sarcoma tumorigenesis and metastasis
Communications Biology (2023)