Abstract
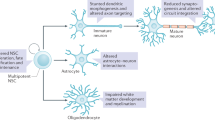
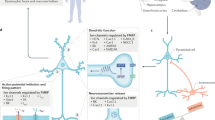
Fragile X syndrome (FXS) is the leading inherited form of intellectual disability and autism spectrum disorder, and patients can present with severe behavioural alterations, including hyperactivity, impulsivity and anxiety, in addition to poor language development and seizures. FXS is a trinucleotide repeat disorder, in which >200 repeats of the CGG motif in FMR1 leads to silencing of the gene and the consequent loss of its product, fragile X mental retardation 1 protein (FMRP). FMRP has a central role in gene expression and regulates the translation of potentially hundreds of mRNAs, many of which are involved in the development and maintenance of neuronal synaptic connections. Indeed, disturbances in neuroplasticity is a key finding in FXS animal models, and an imbalance in inhibitory and excitatory neuronal circuits is believed to underlie many of the clinical manifestations of this disorder. Our knowledge of the proteins that are regulated by FMRP is rapidly growing, and this has led to the identification of multiple targets for therapeutic intervention, some of which have already moved into clinical trials or clinical practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Myrick, L. K. et al. Fragile X syndrome due to a missense mutation. Eur. J. Hum. Genet. 22, 1185–1189 (2014).
Quartier, A. et al. Intragenic FMR1 disease-causing variants: a significant mutational mechanism leading to fragile-X syndrome. Eur. J. Hum. Genet. 25, 423–431 (2017). This paper provided an account of new intragenic mutations in FMR1 and an excellent review of the phenotypes associated with previously reported mutations in FMR1.
Hagerman, R. J. in Fragile X Syndrome: Diagnosis, Treatment and Research (eds Hagerman, R. J. & Hagerman, P. J. ) 3–109 (Johns Hopkins Univ. Press, 2002).
Berry-Kravis, E. et al. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am. J. Intellect. Dev. Disabil. 115, 461–472 (2010).
Hogan, A. L. et al. Autism spectrum disorder symptoms in infants with fragile X syndrome: a prospective case series. J. Autism Dev. Disord. 47, 1628–1644 (2017).
Cordeiro, L., Ballinger, E., Hagerman, R. & Hessl, D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. J. Neurodev. Disord. 3, 57–67 (2011).
Kidd, S. A. et al. Fragile X syndrome: a review of associated medical problems. Pediatrics 134, 995–1005 (2014). This was the first paper to compare frequency of medical problems in a large cohort with FXS with frequencies in the general paediatric population, defining those problems that are more common in FXS; it suggested medical screening and management of these problems for patients with FXS.
Heulens, I. et al. Craniofacial characteristics of fragile X syndrome in mouse and man. Eur. J. Hum. Genet. 21, 816–823 (2013).
Waldstein, G. et al. Fragile X syndrome: skin elastin abnormalities. Birth Defects Orig. Artic. Ser. 23, 103–114 (1987).
Pretto, D. et al. Clinical and molecular implications of mosaicism in FMR1 full mutations. Front. Genet. 5, 318 (2014).
Dyer-Friedman, J. et al. Genetic and environmental influences on the cognitive outcomes of children with fragile X syndrome. J. Am. Acad. Child Adolesc. Psychiatry 41, 237–244 (2002).
Loesch, D. Z., Huggins, R. M. & Hagerman, R. J. Phenotypic variation and FMRP levels in fragile X. Ment. Retard. Dev. Disabil. Res. Rev. 10, 31–41 (2004).
Oostra, B. A. & Hoogeveen, A. in Fragile X Syndrome: Diagnosis, Treatment and Research (eds Hagerman, R. J. & Hagerman, P. J. ) 169–190 (Johns Hopkins Univ. Press, 2002).
Tassone, F. et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am. J. Med. Genet. 84, 250–261 (1999).
Coffee, B. et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am. J. Hum. Genet. 85, 503–514 (2009).
Levesque, S. et al. Screening and instability of FMR1 alleles in a prospective sample of 24,449 mother-newborn pairs from the general population. Clin. Genet. 76, 511–523 (2009).
Fernandez-Carvajal, I. et al. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J. Mol. Diagn. 11, 324–329 (2009).
Song, F. J., Barton, P., Sleightholme, V., Yao, G. L. & Fry-Smith, A. Screening for fragile X syndrome: a literature review and modelling study. Health Technol. Assess. 7, 1–106 (2003).
Maia, N. et al. Contraction of fully expanded FMR1 alleles to the normal range: predisposing haplotype or rare events? J. Hum. Genet. 62, 269–275 (2017).
Hunter, J. et al. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A 164A, 1648–1658 (2014). This was the largest meta-analysis study conducted to assess prevalence estimates of the full mutation and premutation in the total population.
Tassone, F. & Hall, D. A. FXTAS, FXPOI, and Other Premutation Disorders (Springer International Publishing, 2016). This comprehensive book discussed the clinical, epidemiological and molecular issues involved in the premutation disorders that can affect carriers of a premutation throughout their lifespan.
Khandjian, E. W., Corbin, F., Woerly, S. & Rousseau, F. The fragile X mental retardation protein is associated with ribosomes. Nat. Genet. 12, 91–93 (1996).
Tamanini, F. et al. FMRP is associated to the ribosomes via RNA. Hum. Mol. Genet. 5, 809–813 (1996).
Richter, J. D., Bassell, G. J. & Klann, E. Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nat. Rev. Neurosci. 16, 595–605 (2015).
Darnell, J. C. & Klann, E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat. Neurosci. 16, 1530–1536 (2013).
Brown, M. R. et al. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat. Neurosci. 13, 819–821 (2010).
Deng, P. Y. et al. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77, 696–711 (2013).
Alpatov, R. et al. A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response. Cell 157, 869–881 (2014).
Shamay-Ramot, A. et al. Fmrp interacts with Adar and regulates RNA editing, synaptic density and locomotor activity in zebrafish. PLoS Genet. 11, e1005702 (2015).
Akins, M. R., Leblanc, H. F., Stackpole, E. E., Chyung, E. & Fallon, J. R. Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J. Comp. Neurol. 520, 3687–3706 (2012).
Guo, W. et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 17, 559–565 (2011).
Nelson, D. L., Orr, H. T. & Warren, S. T. The unstable repeats — three evolving faces of neurological disease. Neuron 77, 825–843 (2013).
Vershkov, D. & Benvenisty, N. Human pluripotent stem cells in modeling human disorders: the case of fragile X syndrome. Regen. Med. 12, 53–68 (2017).
Colak, D. et al. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science 343, 1002–1005 (2014).
Gerhardt, J. et al. The DNA replication program is altered at the FMR1 locus in fragile X embryonic stem cells. Mol. Cell 53, 19–31 (2014).
Mirkin, S. M. Expandable DNA repeats and human disease. Nature 447, 932–940 (2007).
Zhao, X. N. et al. Mutsβ generates both expansions and contractions in a mouse model of the fragile X-associated disorders. Hum. Mol. Genet. 24, 7087–7096 (2015).
Gholizadeh, S., Halder, S. K. & Hampson, D. R. Expression of fragile X mental retardation protein in neurons and glia of the developing and adult mouse brain. Brain Res. 1596, 22–30 (2015).
Bakker, C. E. & Oostra, B. A. Understanding fragile X syndrome: insights from animal models. Cytogenet. Genome Res. 100, 111–123 (2003).
The Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33 (1994).
Kooy, R. F. Of mice and the fragile X syndrome. Trends Genet. 19, 148–154 (2003).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002). This paper established that loss of FMRP causes exaggerated protein synthesis-dependent mGluRI signalling in the Fmr1-knockout mouse model.
Kaufmann, W. E. et al. Autism spectrum disorder in fragile X syndrome: cooccurring conditions and current treatment. Pediatrics 139, S194–S206 (2017).
Li, J., Pelletier, M. R., Perez Velazquez, J. L. & Carlen, P. L. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell. Neurosci. 19, 138–151 (2002).
Sidorov, M. S., Auerbach, B. D. & Bear, M. F. Fragile X mental retardation protein and synaptic plasticity. Mol. Brain 6, 15 (2013).
Qin, M., Kang, J., Burlin, T. V., Jiang, C. & Smith, C. B. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J. Neurosci. 25, 5087–5095 (2005).
Dolen, G. et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007).
Contractor, A., Klyachko, V. A. & Portera-Cailliau, C. Altered neuronal and circuit excitability in fragile X syndrome. Neuron 87, 699–715 (2015).
Miller, L. J. et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am. J. Med. Genet. 83, 268–279 (1999).
Santoro, M. R., Bray, S. M. & Warren, S. T. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu. Rev. Pathol. 7, 219–245 (2012).
Braat, S. & Kooy, R. F. Fragile X syndrome neurobiology translates into rational therapy. Drug Discov. Today 19, 510–519 (2014).
Pop, A. S., Gomez-Mancilla, B., Neri, G., Willemsen, R. & Gasparini, F. Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology (Berl.) 231, 1217–1226 (2014).
Bear, M. F., Huber, K. M. & Warren, S. T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004).
Pfeiffer, B. E. & Huber, K. M. Current advances in local protein synthesis and synaptic plasticity. J. Neurosci. 26, 7147–7150 (2006).
Sutton, M. A. & Schuman, E. M. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127, 49–58 (2006).
Gkogkas, C. G. et al. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep. 9, 1742–1755 (2014).
Sawicka, K., Pyronneau, A., Chao, M., Bennett, M. V. & Zukin, R. S. Elevated ERK/p90 ribosomal S6 kinase activity underlies audiogenic seizure susceptibility in fragile X mice. Proc. Natl Acad. Sci. USA 113, E6290–E6297 (2016).
Hoeffer, C. A. et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 11, 332–341 (2012).
Braat, S. & Kooy, R. F. Insights into GABAAergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology 88, 48–54 (2015).
Gross, C. et al. Increased expression of the PI3K enhancer PIKE mediates deficits in synaptic plasticity and behavior in fragile X syndrome. Cell Rep. 11, 727–736 (2015).
Sidhu, H., Dansie, L. E., Hickmott, P. W., Ethell, D. W. & Ethell, I. M. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J. Neurosci. 34, 9867–9879 (2014).
Guo, W. et al. Inhibition of GSK3β improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21, 681–691 (2012).
Pasciuto, E. et al. Dysregulated ADAM10-mediated processing of APP during a critical time window leads to synaptic deficits in fragile X syndrome. Neuron 87, 382–398 (2015).
Westmark, C. J. et al. Reversal of fragile X phenotypes by manipulation of AβPP/Aβ levels in Fmr1KO mice. PLoS ONE 6, e26549 (2011).
Tabet, R. et al. Fragile X mental retardation protein (FMRP) controls diacylglycerol kinase activity in neurons. Proc. Natl Acad. Sci. USA 113, E3619–E3628 (2016). This paper identified the dysregulation of the DAG/PA homeostasis in neurons lacking FMRP as the possible molecular cause of mGluR signalling alteration.
Michaluk, P. et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 124, 3369–3380 (2011).
Dziembowska, M. et al. High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am. J. Med Genet. A 161A, 1897–1903 (2013).
Bilousova, T. V. et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 46, 94–102 (2009).
Rotschafer, S. E., Trujillo, M. S., Dansie, L. E., Ethell, I. M. & Razak, K. A. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of fragile X syndrome. Brain Res. 1439, 7–14 (2012).
Gantois, I. et al. Metformin ameliorates core deficits in a mouse model of fragile X syndrome. 23, 674–677 (2017).
Imai, S., Kai, M., Yasuda, S., Kanoh, H. & Sakane, F. Identification and characterization of a novel human type II diacylglycerol kinase, DGKκ. J. Biol. Chem. 280, 39870–39881 (2005).
Sakane, F., Imai, S., Kai, M., Yasuda, S. & Kanoh, H. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases. Curr. Drug Targets 9, 626–640 (2008).
van der Zanden, L. F. et al. Common variants in DGKK are strongly associated with risk of hypospadias. Nat. Genet. 43, 48–50 (2011).
Kim, K., Yang, J. & Kim, E. Diacylglycerol kinases in the regulation of dendritic spines. J. Neurochem. 112, 577–587 (2010).
Hall, S. S., Lightbody, A. A., Hirt, M., Rezvani, A. & Reiss, A. L. Autism in fragile X syndrome: a category mistake? J. Am. Acad. Child Adolesc. Psychiatry 49, 921–933 (2010).
Mouslech, Z. & Valla, V. Endocannabinoid system: an overview of its potential in current medical practice. Neuro Endocrinol. Lett. 30, 153–179 (2009).
Pacher, P., Batkai, S. & Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462 (2006).
Zhang, L. & Alger, B. E. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J. Neurosci. 30, 5724–5729 (2010).
Maccarrone, M. et al. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology 35, 1500–1509 (2010).
Busquets-Garcia, A. et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med. 19, 603–607 (2013).
Jung, K. M. et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Commun. 3, 1080 (2012).
Myrick, L. K. et al. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc. Natl Acad. Sci. USA 112, 949–956 (2015).
Ferron, L., Nieto-Rostro, M., Cassidy, J. S. & Dolphin, A. C. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat. Commun. 5, 3628 (2014).
Darnell, J. C. et al. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107, 489–499 (2001).
Brown, V. et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107, 477–487 (2001).
Suhl, J. A., Chopra, P., Anderson, B. R., Bassell, G. J. & Warren, S. T. Analysis of FMRP mRNA target datasets reveals highly associated mRNAs mediated by G-quadruplex structures formed via clustered WGGA sequences. Hum. Mol. Genet. 23, 5479–5491 (2014).
Braat, S. & Kooy, R. F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 86, 1119–1130 (2015). This paper highlighted that the GABAergic system is compromised in a range of related neurodevelopmental disorders, including FXS.
Curia, G., Papouin, T., Seguela, P. & Avoli, M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex 19, 1515–1520 (2009).
Sabanov, V. et al. Impaired GABAergic inhibition in the hippocampus of Fmr1 knockout mice. Neuropharmacology 116, 71–81 (2017).
Olmos-Serrano, J. L. et al. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J. Neurosci. 30, 9929–9938 (2010).
Vislay, R. L. et al. Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome. J. Neurosci. 33, 7548–7558 (2013).
Centonze, D. et al. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol. Psychiatry 63, 963–973 (2008).
Paluszkiewicz, S. M., Olmos-Serrano, J. L., Corbin, J. G. & Huntsman, M. M. Impaired inhibitory control of cortical synchronization in fragile X syndrome. J. Neurophysiol. 106, 2264–2272 (2011).
Gibson, J. R., Bartley, A. F., Hays, S. A. & Huber, K. M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 100, 2615–2626 (2008).
He, Q., Nomura, T., Xu, J. & Contractor, A. The developmental switch in GABA polarity is delayed in fragile X mice. J. Neurosci. 34, 446–450 (2014).
Tyzio, R. et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343, 675–679 (2014).
D’Hulst, C. & Kooy, R. F. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 30, 425–431 (2007).
Lozano, R., Hare, E. B. & Hagerman, R. J. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr. Dis. Treat. 10, 1769–1779 (2014).
Chang, S. et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat. Chem. Biol. 4, 256–263 (2008).
Olmos-Serrano, J. L., Corbin, J. G. & Burns, M. P. The GABAA receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev. Neurosci. 33, 395–403 (2011).
Reddy, D. S. & Estes, W. A. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol. Sci. 37, 543–561 (2016).
Tassone, F. Advanced technologies for the molecular diagnosis of fragile X syndrome. Expert Rev. Mol. Diagn. 15, 1465–1473 (2015).
Braat, S. et al. The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 14, 2985–2995 (2015).
Kashima, R. et al. Augmented noncanonical BMP type II receptor signaling mediates the synaptic abnormality of fragile X syndrome. Sci. Signal. 9, ra58 (2016).
Hagerman, P. Fragile X-associated tremor/ataxia syndrome (FXTAS): pathology and mechanisms. Acta Neuropathol. 126, 1–19 (2013).
Hazlett, H. C. et al. Trajectories of early brain volume development in fragile X syndrome and autism. J. Am. Acad. Child Adolesc. Psychiatry 51, 921–933 (2012).
Reiss, A. L., Abrams, M. T., Greenlaw, R., Freund, L. & Denckla, M. B. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat. Med. 1, 159–167 (1995).
Reiss, A. L., Patel, S., Kumar, A. J. & Freund, L. Preliminary communication: neuroanatomical variations of the posterior fossa in men with the fragile X (Martin–Bell) syndrome. Am. J. Med. Genet. 31, 407–414 (1988).
Mostofsky, S. H. et al. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology 50, 121–130 (1998).
Gothelf, D. et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP). Ann. Neurol. 63, 40–51 (2008).
Hoeft, F. et al. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch. Gen. Psychiatry 65, 1087–1097 (2008).
Wang, J. Y. et al. Abnormal trajectories in cerebellum and brainstem volumes in carriers of the fragile X premutation. Neurobiol. Aging 55, 11–19 (2017).
Shelton, A. L. et al. White matter microstructure, cognition, and molecular markers in fragile X premutation females. Neurology 88, 2080–2088 (2017).
Eliez, S., Blasey, C. M., Freund, L. S., Hastie, T. & Reiss, A. L. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain 124, 1610–1618 (2001).
Hazlett, H. C. et al. Teasing apart the heterogeneity of autism: same behavior, different brains in toddlers with fragile X syndrome and autism. J. Neurodev. Disord. 1, 81–90 (2009).
Bruno, J. L. et al. Altered brain network segregation in fragile X syndrome revealed by structural connectomics. Cereb. Cortex 27, 2249–2259 (2017).
Bruno, J. L. et al. Aberrant basal ganglia metabolism in fragile X syndrome: a magnetic resonance spectroscopy study. J. Neurodev. Disord. 5, 20 (2013).
Wolff, J. J., Hazlett, H. C., Lightbody, A. A., Reiss, A. L. & Piven, J. Repetitive and self-injurious behaviors: associations with caudate volume in autism and fragile X syndrome. J. Neurodev. Disord. 5, 12 (2013).
Reiss, A. L., Lee, J. & Freund, L. Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology 44, 1317–1324 (1994).
Kates, W. R., Abrams, M. T., Kaufmann, W. E., Breiter, S. N. & Reiss, A. L. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 75, 31–48 (1997).
Jakala, P. et al. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. J. Clin. Invest. 100, 331–338 (1997).
Hall, S. S., Dougherty, R. F. & Reiss, A. L. Profiles of aberrant white matter microstructure in fragile X syndrome. Neuroimage Clin. 11, 133–138 (2016).
Hall, S. S., Jiang, H., Reiss, A. L. & Greicius, M. D. Identifying large-scale brain networks in fragile X syndrome. JAMA Psychiatry 70, 1215–1223 (2013).
Garrett, A. S., Menon, V., MacKenzie, K. & Reiss, A. L. Here's looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Arch. Gen. Psychiatry 61, 281–288 (2004).
Watson, C., Hoeft, F., Garrett, A. S., Hall, S. S. & Reiss, A. L. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch. Gen. Psychiatry 65, 1315–1323 (2008).
Holsen, L. M., Dalton, K. M., Johnstone, T. & Davidson, R. J. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage 43, 592–604 (2008).
Kwon, H. et al. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: relation to behavioral and molecular measures. Am. J. Psychiatry 158, 1040–1051 (2001).
Menon, V., Leroux, J., White, C. D. & Reiss, A. L. Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proc. Natl Acad. Sci. USA 101, 3615–3620 (2004).
Rivera, S. M., Menon, V., White, C. D., Glaser, B. & Reiss, A. L. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Hum. Brain Mapp. 16, 206–218 (2002).
Klabunde, M. et al. Examining the neural correlates of emergent equivalence relations in fragile X syndrome. Psychiatry Res. 233, 373–379 (2015).
Tamm, L., Menon, V., Johnston, C. K., Hessl, D. R. & Reiss, A. L. fMRI study of cognitive interference processing in females with fragile X syndrome. J. Cogn. Neurosci. 14, 160–171 (2002).
Hoeft, F. et al. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum. Brain Mapp. 28, 543–554 (2007).
Rajan-Babu, I. S. & Chong, S. S. Molecular correlates and recent advancements in the diagnosis and screening of FMR1-related disorders. Genes 7 E87 (2016).
Yrigollen, C. M. et al. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet. Med. 14, 729–736 (2012).
Nolin, S. L. et al. Fragile X AGG analysis provides new risk predictions for 45–69 repeat alleles. Am. J. Med Genet. A 161A, 771–778 (2013).
Yrigollen, C. M. et al. AGG interruptions and maternal age affect FMR1 CGG repeat allele stability during transmission. J. Neurodev. Disord. 6, 24 (2014). This large study aimed to determine the predicted risk to expansion to a full mutation during maternal transmission, and it identified CGG repeat number, AGG interruptions and maternal age as the main players.
Bailey, D. B. Jr, Raspa, M., Bishop, E. & Holiday, D. No change in the age of diagnosis for fragile X syndrome: findings from a national parent survey. Pediatrics 124, 527–533 (2009).
Tassone, F. et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 4, 100 (2012).
Harris, S. W. et al. Autism profiles of males with fragile X syndrome. Am. J. Ment. Retard. 113, 427–438 (2008).
Kaufmann, W. E. et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am. J. Med Genet. A 129A, 225–234 (2004).
Yuhas, J. et al. High-risk fragile X screening in Guatemala: use of a new blood spot polymerase chain reaction technique. Genet. Test. Mol. Biomarkers 13, 855–859 (2009).
Winarni, T. I. et al. Identification of expanded alleles of the FMR1 gene among high-risk population in Indonesia by using blood spot screening. Genet. Test. Mol. Biomarkers 16, 162–166 (2012).
Kanwal, M. et al. Molecular diagnosis of fragile X syndrome in subjects with intellectual disability of unknown origin: implications of its prevalence in regional Pakistan. PLoS ONE 10, e0122213 (2015).
McConkie-Rosell, A. et al. Recommendations from multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. J. Genet. Couns. 16, 593–606 (2007).
Berman, R. F. et al. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J. Neurodev. Disord. 6, 25 (2014).
Polussa, J., Schneider, A. & Hagerman, R. Molecular advances leading to treatment implications for fragile X premutation carriers. Brain Disord. Ther. 3 1000119 (2014).
Visootsak, J. et al. Climbing the branches of a family tree: diagnosis of fragile X syndrome. J. Pediatr. 164, 1292–1295 (2014).
Rogers, S. J. et al. Teaching young nonverbal children with autism useful speech: a pilot study of the Denver Model and PROMPT interventions. J. Autism Dev. Disord. 36, 1007–1024 (2006).
Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 125, e17–23 (2010).
Dawson, G. et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry 51, 1150–1159 (2012).
Braden, M. L. Fragile, Handle with Care: More About Fragile X Syndrome, Adolescents and Adults (Spectra Publishing Co., 2000).
Hills Epstein, J. L., Riley, K. & Sobesky, W. in Fragile X Syndrome: Diagnosis, Treatment, and Research (eds Hagerman, R. J. & Hagerman, P. J. ) 339–362 (Johns Hopkins Univ. Press, 2002).
Hagerman, R. J., Murphy, M. A. & Wittenberger, M. D. A controlled trial of stimulant medication in children with the fragile X syndrome. Am. J. Med. Genet. 30, 377–392 (1988).
Hagerman, R. J. et al. Advances in the treatment of fragile X syndrome. Pediatrics 123, 378–390 (2009).
Wirojanan, J. et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J. Clin. Sleep Med. 5, 145–150 (2009).
Torrioli, M. G. et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am. J. Med. Genet. 87, 366–368 (1999).
Torrioli, M. et al. Treatment with valproic acid ameliorates ADHD symptoms in fragile X syndrome boys. Am. J. Med Genet. A 152A, 1420–1427 (2010).
Greiss Hess, L. et al. A randomized, double-blind, placebo-controlled trial of low-dose sertraline in young children with fragile X syndrome. J. Dev. Behav. Pediatr. 37, 619–628 (2016). This paper used a low dose of sertraline in young children (2–6 years of age) with FXS and found significant improvement compared with placebo in development, including visual reception, fine motor coordination, composite cognitive score and, in those with FXS plus ASD, overall expressive language score. This work suggested that early treatment with low-dose sertraline is beneficial and can be used clinically.
Erickson, C. A., Stigler, K. A., Posey, D. J. & McDougle, C. J. Aripiprazole in autism spectrum disorders and fragile X syndrome. Neurotherapeutics 7, 258–263 (2010).
Hersh, J. H. & Saul, R. A. Health supervision for children with fragile X syndrome. Pediatrics 127, 994–1006 (2011).
Kronk, R. et al. Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep 33, 679–687 (2010).
McLennan, Y., Polussa, J., Tassone, F. & Hagerman, R. Fragile X syndrome. Curr. Genomics 12, 216–224 (2011).
Nowicki, S. T. et al. The Prader–Willi phenotype of fragile X syndrome. J. Dev. Behav. Pediatr. 28, 133–138 (2007).
Dy, A. B. C. et al. Metformin as targeted treatment in fragile X syndrome. Clin. Genet.http://dx.doi.org/10.1111/cge.13039 (2017).
Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl Med. 8, 321ra325 (2016). This was hitherto the largest phase IIb clinical trial and illustrated the complexity of FXS pathology and the difficulty of the translation of human treatments validated in mouse models.
Jacquemont, S. et al. Epigenetic modification of the FMR1 gene in fragile X. syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci. Transl Med. 3, 64ra61 (2011).
Wang, H. et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron 59, 634–647 (2008).
Curie, A. et al. Placebo responses in genetically determined intellectual disability: a meta-analysis. PLoS ONE 10, e0133316 (2015).
Budimirovic, D. B. et al. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J. Neurodev. Disord. 9, 14 (2017).
Henderson, C. et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci. Transl Med. 4, 152ra128 (2012).
Berry-Kravis, E. M. et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl Med. 4, 152ra127 (2012).
Sansone, S. M. et al. Psychometric study of the Aberrant Behavior Checklist in fragile X syndrome and implications for targeted treatment. J. Autism Dev. Disord. 42, 1377–1392 (2012).
Berry-Kravis, E. et al. Arbaclofen in fragile X syndrome: results of phase 3 trials. J. Neurodev. Disord. 9, 3 (2017).
Erickson, C. A. et al. Impact of acamprosate on behavior and brain-derived neurotrophic factor: an open-label study in youth with fragile X syndrome. Psychopharmacology (Berl.) 228, 75–84 (2013).
Erickson, C. A. et al. Impact of acamprosate on plasma amyloid-beta precursor protein in youth: a pilot analysis in fragile X syndrome-associated and idiopathic autism spectrum disorder suggests a pharmacodynamic protein marker. J. Psychiatr. Res. 59, 220–228 (2014).
Berry-Kravis, E., Rubin, J., Harary, E. & Daniely, Y. A 6-week, randomized, multicenter, double-blind, parallel, flexed- and fixed-dose study of MDX (metadoxine extended-release; MG01CI) compared with placebo in adolescents and adults with fragile X syndrome. AACAPhttp://files.shareholder.com/downloads/AMDA-1SVKDP/0x0x858015/E85D78F1-33D2-46F4-B4C7-96472D1F9A22/AACAP_AL014_poster_final.pdf (2015).
Knox, A. et al. Feasibility, reliability, and clinical validity of the Test of Attentional Performance for Children (KiTAP) in fragile X syndrome (FXS). J. Neurodev. Disord. 4, 2 (2012).
Ligsay, A. et al. A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome. J. Neurodev. Disord. 9, 26 (2017).
McDuffie, A. et al. Distance video-teleconferencing in early intervention. Top. Early Childhood Special Educ. 33, 172–185 (2013).
McDuffie, A. et al. A spoken-language intervention for school-aged boys with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 121, 236–265 (2016).
McDuffie, A. et al. Early language intervention using distance video-teleconferencing: a pilot study of young boys with fragile X syndrome and their mothers. Am. J. Speech Lang. Pathol. 25, 46–66 (2016).
Schneider, A. et al. Electrocortical changes associated with minocycline treatment in fragile X syndrome. J. Psychopharmacol. 27, 956–963 (2013).
Farzin, F., Scaggs, F., Hervey, C., Berry-Kravis, E. & Hessl, D. Reliability of eye tracking and pupillometry measures in individuals with fragile X syndrome. J. Autism Dev. Disord. 41, 1515–1522 (2011).
Berry-Kravis, E. et al. Sensitivity of the KiTAP executive function battery and an eye tracking paradigm to effects of AFQ056 in fragile X syndrome. Ann. Neurol. 80, S413 (2016).
Paribello, C. et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 10, 91 (2010).
Utari, A. et al. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am. J. Intellect. Dev. Disabil. 115, 433–443 (2010).
Leigh, M. J. et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J. Dev. Behav. Pediatr. 34, 147–155 (2013).
Monyak, R. E. et al. Insulin signaling misregulation underlies circadian and cognitive deficits in a Drosophila fragile X model. Mol. Psychiatry 22, 1140–1148 (2017).
Osterweil, E. K. et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron 77, 243–250 (2013).
Caku, A., Pellerin, D., Bouvier, P., Riou, E. & Corbin, F. Effect of lovastatin on behavior in children and adults with fragile X syndrome: an open-label study. Am. J. Med Genet. A 164A, 2834–2842 (2014).
Pellerin, D. et al. Lovastatin corrects ERK pathway hyperactivation in fragile X syndrome: potential of platelet's signaling cascades as new outcome measures in clinical trials. Biomarkers 21, 497–508 (2016).
Deacon, R. M. et al. NNZ-2566, a novel analog of (1–3) IGF-1, as a potential therapeutic agent for fragile X syndrome. Neuromolecular Med. 17, 71–82 (2015).
Deacon, R. M. et al. Nrf2, a novel therapeutic target in fragile X syndrome is modulated by NNZ2566. Genes Brain Behav.http://dx.doi.org/10.1111/gbb.12373 (2017).
Berry-Kravis, E. et al. The treatment of fragile X syndrome with Trofinetide (NNZ-2566). Ann. Neurol. 80, S412 (2016).
Bailey, D. B., Raspa, M. & Olmsted, M. G. Using a parent survey to advance knowledge about the nature and consequences of fragile X syndrome. Am. J. Intellect. Dev. Disabil. 115, 447–460 (2010).
Chevreul, K. et al. Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur. J. Health Econom. 17 (Suppl. 1), 43–52 (2016). This paper described the burden and costs of FXS and suggested key outcomes that should change as a function of appropriate treatment.
Bailey, D. B. Jr ., Raspa, M., Olmsted, M. & Holiday, D. B. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am. J. Med Genet. A 146A, 2060–2069 (2008).
Chevreul, K., Berg Brigham, K., Brunn, M., des Portes, V. & Network, B.-R. R. Fragile X syndrome: economic burden and health-related quality of life of patients and caregivers in France. J. Intellect. Disabil. Res. 59, 1108–1120 (2015).
Bailey, D. B. Jr, Sideris, J., Roberts, J. & Hatton, D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: a multidimensional analysis. Am. J. Med Genet. A 146A, 720–729 (2008).
Wheeler, A. C., Skinner, D. G. & Bailey, D. B. Perceived quality of life in mothers of children with fragile X syndrome. Am. J. Ment. Retard. 113, 159–177 (2008).
Raspa, M., Bailey, D. B. Jr, Bann, C. & Bishop, E. Modeling family adaptation to fragile X syndrome. Am. J. Intellect. Dev. Disabil. 119, 33–48 (2014).
Bailey, D. B. Jr . et al. Health and economic consequences of fragile X syndrome for caregivers. J. Dev. Behav. Pediatr. 33, 705–712 (2012).
Roberts, J. E. et al. Trajectory and predictors of depression and anxiety disorders in mothers with the FMR1 premutation. Biol. Psychiatry 79, 850–857 (2016). This paper provided the first insights into the longitudinal effects of premutation carrier status on depression and anxiety disorders.
Ouyang, L., Grosse, S., Raspa, M. & Bailey, D. Employment impact and financial burden for families of children with fragile X syndrome: findings from the National Fragile X Survey. J. Intellect. Disabil. Res. 54, 918–928 (2010).
Ouyang, L. et al. A comparison of family financial and employment impacts of fragile X syndrome, autism spectrum disorders, and intellectual disability. Res. Dev. Disabil. 35, 1518–1527 (2014).
Cross, J. et al. Caregiver preferences for the treatment of males with fragile X syndrome. J. Dev. Behav. Pediatr. 37, 71–79 (2016).
Berry-Kravis, E. et al. Development of an expressive language sampling procedure in fragile X syndrome: a pilot study. J. Dev. Behav. Pediatr. 34, 245–251 (2013).
Hessl, D. et al. The NIH Toolbox Cognitive Battery for intellectual disabilities: three preliminary studies and future directions. J. Neurodev. Disord. 8, 35 (2016).
Sansone, S. M. et al. Improving IQ measurement in intellectual disabilities using true deviation from population norms. J. Neurodev. Disord. 6, 16 (2014).
Sherman, S. et al. FORWARD: a registry and longitudinal clinical database to study fragile X syndrome. Pediatrics 139, S3 (2017).
Rogers, S. J. et al. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J. Autism Dev. Disord. 44, 2981–2995 (2014).
Au, J. et al. A feasibility trial of Cogmed working memory training in fragile X syndrome. J. Pediatr. Genet. 3, 147–156 (2014).
Park, C. Y. et al. Reversion of FMR1 methylation and silencing by editing the triplet repeats in fragile X iPSC-derived neurons. Cell Rep. 13, 234–241 (2015).
Tassone, F. et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 13, 555–562 (2007).
Tassone, F. et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15 (2000).
Hagerman, R. J. & Hagerman, P. Fragile X-associated tremor/ataxia syndrome — features, mechanisms and management. Nat. Rev. Neurol. 12, 403–412 (2016).
Sherman, S., Pletcher, B. A. & Driscoll, D. A. Fragile X syndrome: diagnostic and carrier testing. Genet. Med. 7, 584–587 (2005).
Hagerman, R. & Hagerman, P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 12, 786–798 (2013).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Ascano, M. Jr et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492, 382–386 (2012).
Miyashiro, K. Y. et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37, 417–431 (2003).
Tang, B. et al. Fmr1 deficiency promotes age-dependent alterations in the cortical synaptic proteome. Proc. Natl Acad. Sci. USA 112, E4697–E4706 (2015).
Schenck, A., Bardoni, B., Moro, A., Bagni, C. & Mandel, J. L. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc. Natl Acad. Sci. USA 98, 8844–8849 (2001).
Napoli, I. et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, 1042–1054 (2008).
Jin, P. et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 7, 113–117 (2004).
Muddashetty, R. S. et al. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell 42, 673–688 (2011).
Bechara, E. G. et al. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 7, e16 (2009).
Chen, E., Sharma, M. R., Shi, X., Agrawal, R. K. & Joseph, S. Fragile x mental retardation protein regulates translation by binding directly to the ribosome. Mol. Cell 54, 407–417 (2014).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Boland, M. J. et al. Molecular analyses of neurogenic defects in a human pluripotent stem cell model of fragile X syndrome. Brain 140, 582–598 (2017).
Fatemi, S. H., Folsom, T. D., Rooney, R. J. & Thuras, P. D. mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP–mGluR5 signaling pathway. Transl Psychiatry 3, e271 (2013).
Fatemi, S. H., Kneeland, R. E., Liesch, S. B. & Folsom, T. D. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr. Res. 124, 246–247 (2010).
McDuffie, A., Thurman, A. J., Hagerman, R. J. & Abbeduto, L. Symptoms of Autism in males with fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J. Autism Dev. Disord. 45, 1925–1937 (2015).
Thurman, A. J., McDuffie, A., Hagerman, R. & Abbeduto, L. Psychiatric symptoms in boys with fragile X syndrome: a comparison with nonsyndromic autism spectrum disorder. Res. Dev. Disabil. 35, 1072–1086 (2014).
Talisa, V. B., Boyle, L., Crafa, D. & Kaufmann, W. E. Autism and anxiety in males with fragile X syndrome: an exploratory analysis of neurobehavioral profiles from a parent survey. Am. J. Med Genet. A 164A, 1198–1203 (2014).
Hardiman, R. L. & Bratt, A. Hypothalamic–pituitary–adrenal axis function in fragile X syndrome and its relationship to behaviour: a systematic review. Physiol. Behav. 167, 341–353 (2016).
Hessl, D., Rivera, S. M. & Reiss, A. L. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 10, 17–24 (2004).
Thurman, A. J., McDuffie, A., Hagerman, R. J., Josol, C. K. & Abbeduto, L. Language skills of males with fragile X syndrome or nonsyndromic autism spectrum disorder. J. Autism Dev. Disord. 47, 728–743 (2017).
Oberman, L. M. et al. Abnormal mechanisms of plasticity and metaplasticity in autism spectrum disorders and fragile X syndrome. J. Child Adolesc. Psychopharmacol. 26, 617–624 (2016).
Hartley, S. L. et al. Exploring the adult life of men and women with fragile X syndrome: results from a national survey. Am. J. Intellect. Dev. Disabil. 116, 16–35 (2011).
Wheeler, A. C., Raspa, M., Bishop, E. & Bailey, D. B. Jr . Aggression in fragile X syndrome. J. Intellect. Disabil. Res. 60, 113–125 (2016).
Raspberry, K. A. & Skinner, D. Negotiating desires and options: how mothers who carry the fragile X gene experience reproductive decisions. Soc. Sci. Med. 72, 992–998 (2011).
Raspa, M., Edwards, A., Wheeler, A. C., Bishop, E. & Bailey, D. B. Jr . Family communication and cascade testing for fragile X syndrome. J. Genet. Couns. 25, 1075–1084 (2016).
Wheeler, A. C. et al. Associated features in females with an FMR1 premutation. J. Neurodev. Disord. 6, 30 (2014).
Wheeler, A. C. et al. Health and reproductive experiences of women with an FMR1 premutation with and without fragile X premature ovarian insufficiency. Front. Genet. 5, 300 (2014).
Cornish, K., Cole, V., Longhi, E., Karmiloff-Smith, A. & Scerif, G. Mapping developmental trajectories of attention and working memory in fragile X syndrome: developmental freeze or developmental change? Dev. Psychopathol. 25, 365–376 (2013).
Cornish, K., Scerif, G. & Karmiloff-Smith, A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex 43, 672–685 (2007).
Musumeci, S. A. et al. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia 40, 1092–1099 (1999).
Roberts, J. E. et al. Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. J. Neurodev. Disord. 1, 283–291 (2009).
de Vries, B. B. et al. Mental status of females with an FMR1 gene full mutation. Am. J. Hum. Genet. 58, 1025–1032 (1996).
Utari, A. et al. Aging in fragile X syndrome. J. Neurodev. Disord. 2, 70–76 (2010).
Abe, T. et al. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 267, 13361–13368 (1992).
Banko, J. L., Hou, L., Poulin, F., Sonenberg, N. & Klann, E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 26, 2167–2173 (2006).
Tanimura, A. et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010).
Deng, P. Y. & Klyachko, V. A. Increased persistent sodium current causes neuronal hyperexcitability in the entorhinal cortex of Fmr1 knockout mice. Cell Rep. 16, 3157–3166 (2016).
Osterweil, E. K., Krueger, D. D., Reinhold, K. & Bear, M. F. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J. Neurosci. 30, 15616–15627 (2010).
Sharma, A. et al. Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 30, 694–702 (2010).
Gross, C. et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci. 30, 10624–10638 (2010).
Chen, L. Y. et al. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J. Neurosci. 30, 10977–10984 (2010).
Almena, M. & Merida, I. Shaping up the membrane: diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 36, 593–603 (2011).
Ghosh, S. & Bell, R. M. Regulation of Raf-1 kinase by interaction with the lipid second messenger, phosphatidic acid. Biochem. Soc. Trans. 25, 561–565 (1997).
Stace, C. et al. PA binding of phosphatidylinositol 4-phosphate 5-kinase. Adv. Enzyme Regul. 48, 55–72 (2008).
Avila-Flores, A., Santos, T., Rincon, E. & Merida, I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J. Biol. Chem. 280, 10091–10099 (2005).
Gantois, I. et al. Expression profiling suggests underexpression of the GABAA receptor subunit delta in the fragile X knockout mouse model. Neurobiol. Dis. 21, 346–357 (2006).
D’Hulst, C. et al. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 1121, 238–245 (2006). This was the first paper to demonstrate convincingly that GABAergic abnormalities underlie FXS.
Hong, A., Zhang, A., Ke, Y., El Idrissi, A. & Shen, C. H. Downregulation of GABAA beta subunits is transcriptionally controlled by Fmr1p. J. Mol. Neurosci. 46, 272–275 (2011).
El Idrissi, A. et al. Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett. 377, 141–146 (2005).
Gatto, C. L., Pereira, D. & Broadie, K. GABAergic circuit dysfunction in the Drosophila fragile X syndrome model. Neurobiol. Dis. 65, 142–159 (2014).
Adusei, D. C., Pacey, L. K., Chen, D. & Hampson, D. R. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59, 167–171 (2010).
Kratovac, S. & Corbin, J. G. Developmental changes in expression of inhibitory neuronal proteins in the fragile X syndrome mouse basolateral amygdala. Brain Res. 1537, 69–78 (2013).
D’Hulst, C. et al. Positron emission tomography (PET) quantification of GABAA receptors in the brain of fragile X patients. PLoS ONE 10, e0131486 (2015).
Kang, J. Y. et al. Deficits in the activity of presynaptic γ-aminobutyric acid type B receptors contribute to altered neuronal excitability in fragile X syndrome. J. Biol. Chem. 292, 6621–6632 (2017).
D’Hulst, C. et al. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res. 1253, 176–183 (2009).
Davidovic, L. et al. A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res. 21, 2190–2202 (2011).
Paluszkiewicz, S. M., Martin, B. S. & Huntsman, M. M. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev. Neurosci. 33, 349–364 (2011).
Gross, C., Hoffmann, A., Bassell, G. J. & Berry-Kravis, E. M. Therapeutic strategies in fragile X syndrome: from bench to bedside and back. Neurotherapeutics 12, 584–608 (2015). This was a comprehensive review of all preclinical work and targeted treatments in clinical trials for FXS, including outcome measures used and measures showing change.
Hoffmann, A. & Berry-Kravis, E. in Neuronal and Synaptic Dysfunction in Autism Spectrum Disorder and Intellectual Disability (eds Sala, C. & Verpelli, C. ) 325–346 (Academic Press, 2016).
Acknowledgements
This work was supported by the following grants. R.J.H., P.J.H. and F.T. were funded by US Health Resources and Services Administration (HRSA) grant R40MC27701, National Institute of Child Health and Human Development grant R01HD036071, HRSA grant R40MC22641, Department of Defense grant PR101054, MIND Institute Intellectual and Developmental Disability Research Center U54HD07912. H.M. and L.M. are funded by Agence Nationale de la Recherche (ANR-12-BSV8-0022), Fondation Jérôme Lejeune funding, FRAXA Research Foundation and grant ANR-10-LABX-0030-INRT, a French State fund managed by the Agence Nationale de la Recherche under the frame programme Investissements d’Avenir ANR-10-IDEX-0002-02 to H.M. and J.L.M. R.F.K. is funded by grants from the Research Foundation Flanders, FRAXA Research Foundation and the Fondation Jérôme Lejeune. H.C.H. was funded by NICHD R01HD059854. The authors thank L. Makhoul for help with preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Introduction (R.J.H. and P.J.H.); Epidemiology (F.T.); Mechanisms/pathophysiology (J.L.M., H.M., R.F.K., N.S., H.C.H. and P.J.H.); Diagnosis, screening and prevention (R.J.H. and F.T.); Management (E.B.-K. and R.J.H.); Quality of life (D.B.B.); Outlook (R.J.H., E.B.-K. and P.J.H); Overview of Primer (R.J.H. and P.J.H.).
Corresponding author
Ethics declarations
Competing interests
R.J.H. has received funding from Novartis, Roche, Alcobra, Neuren and Marinus for clinical trials in FXS and has consulted with Zynerba for clinical trials in FXS. E.B.-K. has received funding from Novartis, Roche, Alcobra, Neuren, Cydan, Fulcrum, GW, Marinus, Edison and Neurotrope Pharmaceuticals to consult on trial design and development strategies and/or conduct clinical trials in FXS, and from Asuragen Inc. to develop testing standards for FMR1 testing. D.B.B. received funding from The John Merck Fund to plan a fragile X newborn screening study. R.F.K. has received logistic support and ganaxolone treatment funding from Marinus Pharmaceuticals to conduct a clinical trial in FXS. F.T. has received funding for molecular studies in FXS from Asuragen. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Hagerman, R., Berry-Kravis, E., Hazlett, H. et al. Fragile X syndrome. Nat Rev Dis Primers 3, 17065 (2017). https://doi.org/10.1038/nrdp.2017.65
Published:
DOI: https://doi.org/10.1038/nrdp.2017.65
This article is cited by
-
FMRP-mediated spatial regulation of physiologic NMD targets in neuronal cells
Genome Biology (2024)
-
Sex-specific modulation of early life vocalization and cognition by Fmr1 gene dosage in a mouse model of Fragile X Syndrome
Biology of Sex Differences (2024)
-
mGluR7 allosteric modulator AMN082 corrects protein synthesis and pathological phenotypes in FXS
EMBO Molecular Medicine (2024)
-
Multi-level profiling of the Fmr1 KO rat unveils altered behavioral traits along with aberrant glutamatergic function
Translational Psychiatry (2024)
-
Cannabidiol (Epidyolex®) for severe behavioral manifestations in patients with tuberous sclerosis complex, mucopolysaccharidosis type III and fragile X syndrome: protocol for a series of randomized, placebo-controlled N-of-1 trials
BMC Psychiatry (2024)