Abstract
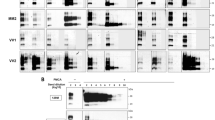
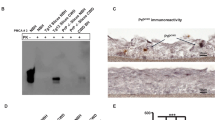
The scrapie prion protein isoform, PrPSc, is a prion-associated marker that seeds the conformational conversion and polymerization of normal protease-sensitive prion protein (PrP-sen). This seeding activity allows ultrasensitive detection of PrPSc using cyclical sonicated amplification (PMCA) reactions and brain homogenate as a source of PrP-sen. Here we describe a much faster seeded polymerization method (rPrP-PMCA) which detects ≥50 ag of hamster PrPSc (≈0.003 lethal dose) within 2–3 d. This technique uses recombinant hamster PrP-sen, which, unlike brain-derived PrP-sen, can be easily concentrated, mutated and synthetically tagged. We generated protease-resistant recombinant PrP fibrils that differed from spontaneously initiated fibrils in their proteolytic susceptibility and by their infrared spectra. This assay could discriminate between scrapie-infected and uninfected hamsters using 2-μl aliquots of cerebral spinal fluid. This method should facilitate the development of rapid, ultrasensitive prion assays and diagnostic tests, in addition to aiding fundamental studies of structure and mechanism of PrPSc formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 95, 13363–13383 (1998).
Caughey, B. & Baron, G.S. Prions and their partners in crime. Nature 443, 803–810 (2006).
Silveira, J.R., Caughey, B. & Baron, G.S. Prion protein and the molecular features of transmissible spongiform encephalopathy agents. Curr. Top. Microbiol. Immunol. 284, 1–50 (2004).
Kocisko, D.A. et al. Cell-free formation of protease-resistant prion protein. Nature 370, 471–474 (1994).
Saborio, G.P., Permanne, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Castilla, J., Saa, P., Hetz, C. & Soto, C. In vitro generation of infectious scrapie prions. Cell 121, 195–206 (2005).
Deleault, N.R. et al. Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. USA 104, 9741–9746 (2007).
Iniguez, V., McKenzie, D., Mirwald, J. & Aiken, J. Strain-specific propagation of PrP(Sc) properties into baculovirus-expressed hamster PrP(C). J. Gen. Virol. 81, 2565–2571 (2000).
Kirby, L., Birkett, C.R., Rudyk, H., Gilbert, I.H. & Hope, J. In vitro cell-free conversion of bacterial recombinant PrP to PrPres as a model for conversion. J. Gen. Virol. 84, 1013–1020 (2003).
Eiden, M. et al. Synergistic and strain-specific effects of bovine spongiform encephalopathy and scrapie prions in the cell-free conversion of recombinant prion protein. J. Gen. Virol. 87, 3753–3761 (2006).
Surewicz, W.K., Jones, E.M. & Apetri, A.C. The emerging principles of mammalian prion propagation and transmissibility barriers: insight from studies in vitro. Acc. Chem. Res. 39, 654–662 (2006).
Baskakov, I.V. & Breydo, L. Converting the prion protein: what makes the protein infectious. Biochim. Biophys. Acta 1772, 692–703 (2007).
Legname, G. et al. Synthetic mammalian prions. Science 305, 673–676 (2004).
Saa, P., Castilla, J. & Soto, C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 281, 35245–35252 (2006).
Saa, P., Castilla, J. & Soto, C. Presymptomatic detection of prions in blood. Science 313, 92–94 (2006).
Raymond, G.J. & Chabry, J. Purification of the pathological isoform of prion protein (PrPSc or PrPres) from transmissible spongiform encephalopathy-affected brain tissue in Techniques in Prion Research (eds., Lehmann, S. & Grassi, J.) 16–26 (Birkhauser Verlag, Basel, 2004).
Peretz, D. et al. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412, 739–743 (2001).
Silveira, J.R. et al. The most infectious prion protein particles. Nature 437, 257–261 (2005).
Caughey, B. et al. Prions and transmissible spongiform encephalopathy (TSE) chemotherapeutics: a common mechanism for anti-TSE compounds? Acc. Chem. Res. 39, 646–653 (2006).
Trevitt, C.R. & Collinge, J. A systematic review of prion therapeutics in experimental models. Brain 129, 2241–2265 (2006).
Pan, K.-M. et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion protein. Proc. Natl. Acad. Sci. USA 90, 10962–10966 (1993).
Hornemann, S., Schorn, C. & Wuthrich, K. NMR structure of the bovine prion protein isolated from healthy calf brains. EMBO Rep. 5, 1159–1164 (2004).
Caughey, B., Raymond, G.J. & Bessen, R.A. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J. Biol. Chem. 273, 32230–32235 (1998).
Caughey, B.W. et al. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30, 7672–7680 (1991).
Xiong, L.W., Raymond, L.D., Hayes, S.F., Raymond, G.J. & Caughey, B. Conformational change, aggregation and fibril formation induced by detergent treatments of cellular prion protein. J. Neurochem. 79, 669–678 (2001).
Bocharova, O.V. et al. Annealing prion protein amyloid fibrils at high temperature results in extension of a proteinase K-resistant core. J. Biol. Chem. 281, 2373–2379 (2006).
Zahn, R., von Schroetter, C. & Wuthrich, K. Human prion proteins expressed in Escherichia coli and purified by high-affinity column refolding. FEBS Lett. 417, 400–404 (1997).
Caughey, B., Raymond, G.J., Ernst, D. & Race, R.E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65, 6597–6603 (1991).
Acknowledgements
We thank K. Meade-White and B. Race for providing us with the CSF samples, G. Raymond for technical assistance, and G. Baron, B. Race and Y. Taguchi for helpful discussions and critical assessment of the manuscript. This research was funded by the Intramural Research Program of the US National Institute of Allergy and Infectious Diseases. V.L.S. also receives funding from a Clinical Fellowship Award from the Alberta Heritage Foundation for Medical Research.
Author information
Authors and Affiliations
Contributions
R.A. initiated the project, conceived and performed most experiments, and helped write the manuscript; R.A.M. prepared rPrP-sen, performed FTIR and edited the manuscript; V.L.S. performed electron microscopy and edited the manuscript; A.G.H. performed supporting experiments and wrote the step-by-step protocol; D.W.D. performed electron microscopy; H.A.O. performed supporting experiments; S.A.P. supervised rPrP-sen preparation and edited the manuscript; B.C. coordinated the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Methods (PDF 701 kb)
Rights and permissions
About this article
Cite this article
Atarashi, R., Moore, R., Sim, V. et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods 4, 645–650 (2007). https://doi.org/10.1038/nmeth1066
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1066
This article is cited by
-
Seed amplification assay of nasal swab extracts for accurate and non-invasive molecular diagnosis of neurodegenerative diseases
Translational Neurodegeneration (2023)
-
Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid
Nature Protocols (2023)
-
Seed amplification and RT-QuIC assays to investigate protein seed structures and strains
Cell and Tissue Research (2023)
-
Role of sialylation of N-linked glycans in prion pathogenesis
Cell and Tissue Research (2023)