Abstract
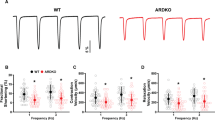
Although much is known about environmental factors that predispose individuals to hypertension and cardiovascular disease, little information is available regarding the genetic and signaling events involved1,2,3. Indeed, few genes associated with the progression of these pathologies have been discovered despite intensive research in animal models and human populations1,2,3. Here we identify Vav3, a GDP-GTP exchange factor that stimulates Rho and Rac GTPases4, as an essential factor regulating the homeostasis of the cardiovascular system. Vav3-deficient mice exhibited tachycardia, systemic arterial hypertension and extensive cardiovascular remodeling. These mice also showed hyperactivity of sympathetic neurons from the time of birth. The high catecholamine levels associated with this condition led to the activation of the renin-angiotensin system, increased levels of kidney-related hormones and the progressive loss of cardiovascular and renal homeostasis. Pharmacological studies with drugs targeting sympathetic and renin-angiotensin responses confirmed the causative role and hierarchy of these events in the development of the Vav3-null mouse phenotype. These observations uncover the crucial role of Vav3 in the regulation of the sympathetic nervous system (SNS) and cardiovascular physiology, and reveal a signaling pathway that could be involved in the pathophysiology of human disease states involving tachycardia and sympathetic hyperactivity with unknown etiologies2,5,6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lifton, R.P., Gharavi, A.G. & Geller, D.S. Molecular mechanisms of human hypertension. Cell 104, 545–556 (2001).
Beevers, G., Lip, G.Y. & O'Brien, E. ABC of hypertension: the pathophysiology of hypertension. Br. Med. J. 322, 912–916 (2001).
Staessen, J.A., Wang, J., Bianchi, G. & Birkenhager, W.H. Essential hypertension. Lancet 361, 1629–1641 (2003).
Movilla, N. & Bustelo, X.R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell. Biol. 19, 7870–7885 (1999).
Palatini, P. & Julius, S. Relevance of heart rate as a risk factor in hypertension. Curr. Hypertens. Rep. 1, 219–224 (1999).
Palatini, P. & Julius, S. The physiological determinants and risk correlations of elevated heart rate. Am. J. Hypertens. 12, 3S–8S (1999).
Bustelo, X.R. Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 20, 1461–1477 (2000).
Turner, M. & Billadeau, D.D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2, 476–486 (2002).
Faccio, R. et al. Vav3 regulates osteoclast function and bone mass. Nat. Med. 11, 284–290 (2005).
Tybulewicz, V.L., Ardouin, L., Prisco, A. & Reynolds, L.F. Vav1: a key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52 (2003).
Fujikawa, K. et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 198, 1595–1608 (2003).
Schmieder, R.E. & Messerli, F.H. Hypertension and the heart. J. Hum. Hypertens. 14, 597–604 (2000).
Intengan, H.D. & Schiffrin, E.L. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38, 581–587 (2001).
Takahashi, N. & Smithies, O. Gene targeting approaches to analyzing hypertension. J. Am. Soc. Nephrol. 10, 1598–1605 (1999).
Remuzzi, G., Perico, N. & Benigni, A. New therapeutics that antagonize endothelin: promises and frustrations. Nat. Rev. Drug Discov. 1, 986–1001 (2002).
DiBona, G.F. The sympathetic nervous system and hypertension: recent developments. Hypertension 43, 147–150 (2004).
Carrasco, G.A. & Van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 463, 235–272 (2003).
Palatini, P. & Julius, S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin. Exp. Hypertens. 26, 637–644 (2004).
Palatini, P., Benetos, A. & Julius, S. Impact of increased heart rate on clinical outcomes in hypertension: implications for antihypertensive drug therapy. Drugs 66, 133–144 (2006).
Turner, M. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7, 451–460 (1997).
Jerkic, M. et al. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 18, 609–611 (2004).
Flores, O. et al. Beneficial effect of the long-term treatment with the combination of an ACE inhibitor and a calcium channel blocker on renal injury in rats with 5/6 nephrectomy. Exp. Nephrol. 6, 39–49 (1998).
Chevillard, C., Brown, N.L., Mathieu, M.N., Laliberte, F. & Worcel, M. Differential effects of oral trandolapril and enalapril on rat tissue angiotensin-converting enzyme. Eur. J. Pharmacol. 147, 23–28 (1988).
Valdivielso, J.M. et al. Renal ischemia in the rat stimulates glomerular nitric oxide synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R771–R779 (2001).
Acknowledgements
We thank J. Tamame, M. Blázquez, T. Iglesias, M. Jerkic and F. Núñez for technical help. We also thank V. Tybulewicz for making available to us his Vav1 knockout mice and M. Dosil for helpful comments on the manuscript. This work was supported by grants from the US National Institutes of Health to X.R.B. and the Spanish Ministry of Education and Science to X.R.B. and J.M.L.-N. V.S. is supported by a European Molecular Biology Organization (EMBO) long-term postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Scheme of the strategy to disrupt the mouse Vav3 gene by homologous recombination. (PDF 344 kb)
Supplementary Fig. 2
Growth rates of Vav3 knockout animals. (PDF 155 kb)
Supplementary Fig. 3
Analysis of the cardiovascular defects found in Vav3−/− animals. (PDF 1420 kb)
Supplementary Fig. 4
Expression levels of the mRNAs for angiotensin II and endothelin receptors. (PDF 71 kb)
Supplementary Fig. 5
Vasopressin and aldosterone levels in Vav3−/−M animals. (PDF 77 kb)
Supplementary Table 1
Cardiovascular parameters in Vav3−/− and Vav1−/− mice. (PDF 94 kb)
Rights and permissions
About this article
Cite this article
Sauzeau, V., Sevilla, M., Rivas-Elena, J. et al. Vav3 proto-oncogene deficiency leads to sympathetic hyperactivity and cardiovascular dysfunction. Nat Med 12, 841–845 (2006). https://doi.org/10.1038/nm1426
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1426
This article is cited by
-
Identification and validation of key biomarkers and potential therapeutic targets for primary open-angle glaucoma
Science China Life Sciences (2023)
-
Genome-wide run of homozygosity analysis reveals candidate genomic regions associated with environmental adaptations of Tibetan native chickens
BMC Genomics (2022)
-
The Rho guanosine nucleotide exchange factors Vav2 and Vav3 modulate epidermal stem cell function
Oncogene (2022)
-
Vav2 pharmaco-mimetic mice reveal the therapeutic value and caveats of the catalytic inactivation of a Rho exchange factor
Oncogene (2020)
-
Vav proteins maintain epithelial traits in breast cancer cells using miR-200c-dependent and independent mechanisms
Oncogene (2019)