Abstract
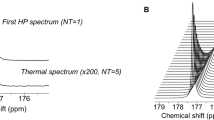
Mutations in isocitrate dehydrogenases 1 and 2 (IDH1 and IDH2) have been shown to be present in most World Health Organization grade 2 and grade 3 gliomas in adults. These mutations are associated with the accumulation of 2-hydroxyglutarate (2HG) in the tumor. Here we report the noninvasive detection of 2HG by proton magnetic resonance spectroscopy (MRS). We developed and optimized the pulse sequence with numerical and phantom analyses for 2HG detection, and we estimated the concentrations of 2HG using spectral fitting in the tumors of 30 subjects. Detection of 2HG correlated with mutations in IDH1 or IDH2 and with increased levels of D-2HG by mass spectrometry of the resected tumors. Noninvasive detection of 2HG may prove to be a valuable diagnostic and prognostic biomarker.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
31 January 2012
In the version of this article initially published online, Craig R. Malloy’s name was incorrectly listed as Craig M. Malloy. Figure 2c was incorrectly labeled as a grade 4 oligodendroglioma; the correct labeling should be grade 2 oligodendroglioma. In reference 13, Ernest, R.R. should have been Ernst, R.R. In the Acknowledgments, one grant was missing: US National Institutes of Health grant RR0584. These errors have been corrected for all versions of this article.
References
Balss, J. et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 116, 597–602 (2008).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med 360, 765–773 (2009).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Figueroa, M.E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Christensen, B.C. et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J. Natl. Cancer Inst. 103, 143–153 (2011).
Parsons, D.W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
von Deimling, A., Korshunov, A. & Hartmann, C. The next generation of glioma biomarkers: MGMT methylation, BRAF fusions and IDH1 mutations. Brain Pathol. 21, 74–87 (2011).
Bottomley, P.A. Selective volume method for performing localized NMR spectroscopy. US Patent 4,480,228 (1984).
Mescher, M., Merkle, H., Kirsch, J., Garwood, M. & Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272 (1998).
Bal, D. & Gryff-Keller, A. 1H and 13C NMR study of 2-hydroxyglutaric acid and lactone. Magn. Reson. Chem. 40, 533–536 (2002).
Krawczyk, H. & Gradowska, W. Characterisation of the 1H and 13C NMR spectra of N-acetylaspartylglutamate and its detection in urine from patients with Canavan disease. J. Pharm. Biomed. Anal. 31, 455–463 (2003).
Govindaraju, V., Young, K. & Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13, 129–153 (2000).
Ernst, R.R., Bodenhausen, G. & Wokaun, A. Principles of nuclear magnetic resonance in one and two dimensions . Ch. 2 (Clarendon Press, Oxford, UK, 1987).
Thompson, R.B. & Allen, P.S. Sources of variability in the response of coupled spins to the PRESS sequence and their potential impact on metabolite quantification. Magn. Reson. Med. 41, 1162–1169 (1999).
Choi, C. et al. Improvement of resolution for brain coupled metabolites by optimized 1H MRS at 7T. NMR Biomed. 23, 1044–1052 (2010).
Gillies, R.J., Raghunand, N., Garcia-Martin, M.L. & Gatenby, R.A. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng. Med. Biol. Mag. 23, 57–64 (2004).
Griffiths, J.R. Are cancer cells acidic?. Br. J. Cancer 64, 425–427 (1991).
McLean, L.A., Roscoe, J., Jorgensen, N.K., Gorin, F.A., & Cala, P.M. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am. J. Physiol. Cell Physiol. 278, C676–C688 (2000).
Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679 (1993).
Mlynarik, V., Gruber, S. & Moser, E. Proton T (1) and T (2) relaxation times of human brain metabolites at 3 tesla. NMR Biomed. 14, 325–331 (2001).
Traber, F., Block, W., Lamerichs, R., Gieseke, J. & Schild, H.H. 1H metabolite relaxation times at 3.0 tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J. Magn. Reson. Imaging 19, 537–545 (2004).
Ganji, S.K. et al. T2 measurement of J-coupled metabolites in the human brain at 3T. NMR Biomed published online, doi:10.1002/nbm.1767 (15 August 2011).
Norton, W.T., Poduslo, S.E. & Suzuki, K. Subacute sclerosing leukoencephalitis. II. Chemical studies including abnormal myelin and an abnormal ganglioside pattern. J. Neuropathol. Exp. Neurol. 25, 582–597 (1966).
Keevil, S.F. et al. Absolute metabolite quantification by in vivo NMR spectroscopy: II. A multicentre trial of protocols for in vivo localised proton studies of human brain. Magn. Reson. Imaging 16, 1093–1106 (1998).
Tong, Z., Yamaki, T., Harada, K. & Houkin, K. In vivo quantification of the metabolites in normal brain and brain tumors by proton MR spectroscopy using water as an internal standard. . Magn. Reson. Imaging 22, 1017–1024 (2004).
Sener, R.N. L-2 hydroxyglutaric aciduria: proton magnetic resonance spectroscopy and diffusion magnetic resonance imaging findings J. Comput. Assist. Tomogr. 27, 38–43 (2003).
Goffette, S.M. et al. L-2-Hydroxyglutaric aciduria: clinical, genetic, and brain MRI characteristics in two adult sisters. Eur. J. Neurol. 13, 499–504 (2006).
Choi, C. et al. Measurement of glycine in the human brain in vivo by 1H-MRS at 3 T: application in brain tumors. Magn. Reson. Med. 66, 609–618 (2011).
Maher, E.A. et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 66, 11502–11513 (2006).
Rakheja, D., Mitui, M., Boriack, R.L. & DeBerardinis, R.J. Isocitrate dehydrogenase 1/2 mutational analyses and 2-hydroxyglutarate measurements in Wilms tumors. Pediatr. Blood Cancer 56, 379–383 (2011).
Rakheja, D. et al. Papillary thyroid carcinoma shows elevated levels of 2-hydroxyglutarate. Tumour Biol. 32, 325–333 (2011).
Acknowledgements
This work was supported by US National Institutes of Health grants RC1NS0760675, R21CA159128 and RR02584 and by the Cancer Prevention Research Institute of Texas grant RP101243-P04. We thank C. Sheppard for expert management of the patient database and for coordinating research scans and tissue samples; S. McNeil for expert human subject care during scanning; C. Foong for expert assistance with pathological analysis of tumor; and R.L. Boriack for expert assistance with 2HG measurements by mass spectrometry.
Author information
Authors and Affiliations
Contributions
C.C. developed the MRS methodology for 2HG detection, designed and performed the magnetic resonance experiments and data analysis, supervised the MRS study, prepared figures and wrote the manuscript. E.A.M. led all aspects of the human study, contributed to data analysis, preparation of the figures and writing of the manuscript. S.K.G. carried out magnetic resonance data acquisition and contributed to data analysis. D.R. performed mass spectrometry analysis on resected tumors and contributed to manuscript preparation. Z.K. synthesized 2HG and prepared a figure. R.J.D. and C.R.M. contributed to the conceptual approach, review of the data and manuscript preparation. K.J.H. and J.M.R. contributed to tumor sample collection and validation, neuropathological evaluation and diagnosis, evaluation of immunohistochemical stains and manuscript preparation. X.-L.Y. and T.M. performed the tissue evaluation of IDH mutations. I.M.-V. and J.M.P. contributed to conceptual analysis. B.E.M. recruited subjects and contributed to clinical data analysis and manuscript preparation. C.J.M. recruited subjects and contributed to manuscript preparation. R.M.B. contributed to the conceptual approach, led the tumor analysis workup, and contributed to data analysis and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Choi, C., Ganji, S., DeBerardinis, R. et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 18, 624–629 (2012). https://doi.org/10.1038/nm.2682
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2682
This article is cited by
-
Imaging cancer metabolism using magnetic resonance
npj Imaging (2024)
-
Exceptionally rare IDH1-mutant adult medulloblastoma with concurrent GNAS mutation revealed by in vivo magnetic resonance spectroscopy and deep sequencing
Acta Neuropathologica Communications (2023)
-
The development of a hiPSC-based platform to identify tissue-dependencies of IDH1 R132H
Cell Death Discovery (2023)
-
Evaluation of isocitrate dehydrogenase mutation in 2021 world health organization classification grade 3 and 4 glioma adult-type diffuse gliomas with 18F-fluoromisonidazole PET
Japanese Journal of Radiology (2023)
-
Clinical applications and prospects of PET imaging in patients with IDH-mutant gliomas
Journal of Neuro-Oncology (2023)