Abstract
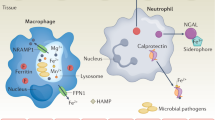
Although iron is required to sustain life, its free concentration and metabolism have to be tightly regulated1. This is achieved through a variety of iron-binding proteins including transferrin and ferritin2. During infection, bacteria acquire much of their iron from the host by synthesizing siderophores that scavenge iron and transport it into the pathogen3,4. We recently demonstrated that enterochelin, a bacterial catecholate siderophore, binds to the host protein lipocalin 2 (ref. 5). Here, we show that this event is pivotal in the innate immune response to bacterial infection. Upon encountering invading bacteria the Toll-like receptors on immune cells stimulate the transcription, translation and secretion of lipocalin 2; secreted lipocalin 2 then limits bacterial growth by sequestrating the iron-laden siderophore. Our finding represents a new component of the innate immune system and the acute phase response to infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weinberg, E. D. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 64, 65–102 (1984)
Andrews, N. C. Iron homeostasis: insights from genetics and animal models. Nature Rev. Genet. 1, 208–217 (2000)
Ratledge, C. & Dover, L. G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54, 881–941 (2000)
Faraldo-Gomez, J. D. & Sansom, M. S. Acquisition of siderophores in gram-negative bacteria. Nature Rev. Mol. Cell Biol. 4, 105–116 (2003)
Goetz, D. H. et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 10, 1033–1043 (2002)
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003)
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002)
Kjeldsen, L., Cowland, J. B. & Borregaard, N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta 1482, 272–283 (2000)
Triebel, S., Blaser, J., Reinke, H. & Tschesche, H. A 25 kDa alpha 2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 314, 386–388 (1992)
Kjeldsen, L., Johnsen, A. H., Sengelov, H. & Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 268, 10425–10432 (1993)
Flower, D. R., North, A. C. & Attwood, T. K. Mouse oncogene protein 24p3 is a member of the lipocalin protein family. Biochem. Biophys. Res. Commun. 180, 69–74 (1991)
Liu, Q., Ryon, J. & Nilsen-Hamilton, M. Uterocalin: a mouse acute phase protein expressed in the uterus around birth. Mol. Reprod. Dev. 46, 507–514 (1997)
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998)
Lien, E. et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274, 33419–33425 (1999)
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000)
Takeuchi, O. et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169, 10–14 (2002)
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001)
Xu, S. & Venge, P. Lipocalins as biochemical markers of disease. Biochim. Biophys. Acta 1482, 298–307 (2000)
Liu, Q. & Nilsen-Hamilton, M. Identification of a new acute phase protein. J. Biol. Chem. 270, 22565–22570 (1995)
Winkelmann, G. Microbial siderophore-mediated transport. Biochem. Soc. Trans. 30, 691–696 (2002)
Yang, J. et al. An iron delivery pathway mediated by a lipocalin. Mol. Cell 10, 1045–1056 (2002)
Suire, S., Stewart, F., Beauchamp, J. & Kennedy, M. W. Uterocalin, a lipocalin provisioning the preattachment equine conceptus: fatty acid and retinol binding properties, and structural characterization. Biochem. J. 356, 369–376 (2001)
Devireddy, L. R., Teodoro, J. G., Richard, F. A. & Green, M. R. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 293, 829–834 (2001)
Hou, Z., Stack, T. D. P., Sunderland, C. J. & Raymond, K. N. Enhanced iron (III) chelation through ligand predisposition: Syntheses, structures and stability of Tris-catecholate enterobactin analogs. Inorg. Chim. Acta 263, 341–355 (1997)
Acknowledgements
We acknowledge T. Hawn and M. Matsumoto for discussions. This work was supported by grants from The Norwegian Research Council (to T.H.F.) and the NIH (to A.A., R.K.S. and K.D.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Targeted disruption of the mouse Lcn2 gene. (JPG 36 kb)
Supplementary Figure 2
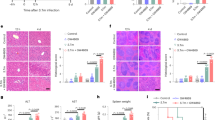
Liver inflammation, spleen iron and infiltrating neutrophils after i.p. infection of lipocalin 2 knockout (-/-) and wild type (+/+) mice with 0.6 ´ 108 c.f.u. E. coli H9049. (JPG 78 kb)
Supplementary Figure 3
Lipocalin 2 deficiency does not affect induction apoptosis upon withdrawal of IL-3 from IL-3-dependent bone marrow cells. (JPG 30 kb)
Supplementary Table 1
Blood and peritoneal cell populations in lipocalin 2-deficient and WT mice. (DOC 23 kb)
Supplementary Table 2
Serum iron and iron binding capacities in lipocalin 2 deficient and wild-type mice. (DOC 20 kb)
Supplementary Figure Legends
Legends to accompany Supplementary Information Figures 1–3. (DOC 22 kb)
Rights and permissions
About this article
Cite this article
Flo, T., Smith, K., Sato, S. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004). https://doi.org/10.1038/nature03104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03104
This article is cited by
-
A potential involvement of LCN2 in isoflurane-induced postoperative cognitive dysfunction
Molecular & Cellular Toxicology (2024)
-
Health disparities in COVID-19: immune and vascular changes are linked to disease severity and persist in a high-risk population in Riverside County, California
BMC Public Health (2023)
-
Interleukin-36 is overexpressed in human sepsis and IL-36 receptor deletion aggravates lung injury and mortality through epithelial cells and fibroblasts in experimental murine sepsis
Critical Care (2023)
-
Lipocalin-2: a therapeutic target to overcome neurodegenerative diseases by regulating reactive astrogliosis
Experimental & Molecular Medicine (2023)
-
Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2
Nature Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.