Abstract
Aims
To evaluate the rate of complications after intravitreal injection of bevacizumab and triamcinolone.
Methods
The clinical interventional case-series study included 5403 intravitreal injections of about 20 mg triamcinolone acetonide (n=1588) or 1.5 mg bevacizumab (n=3818) consecutively performed in the period from 2000 to 2007 by three surgeons for treatment of various intraocular edematous or neovascular diseases. Follow-up after each injection was at least 4 weeks.
Results
An infectious endophthalmitis which necessitated pars plana vitrectomy was detected in two eyes (2/5403 or 0.04±0.03%) from the bevacizumab group. Two eyes (2/5403 or 0.04±0.03%) from the bevacizumab group showed a painless vitreous clouding which subsided after intensified topical antibiotic therapy; one eye (1/5403 or 0.02±0.02%) developed a retinal detachment; and three eyes (3/5403 or 0.06±0.03%) (two eyes from the bevacizumab group) showed a rapidly progressive cataract. The total rate of these complications was 8/5403 (0.15±0.05%). It was statistically independent of the surgeon (P=0.18), the drug injected (P=0.45), and the age of the patients (P=0.87).
Conclusions
Injection-related complications such as infectious endophthalmitis, retinal detachment, and traumatic cataract may occur with a frequency of about 0.15±0.05% after intravitreal injections of bevacizumab or triamcinolone, independently of the drug injected.
Similar content being viewed by others
Main
Since 2000 and 2005, the intravitreal applications of triamcinolone acetonide and bevacizumab/ranibizumab have markedly increased in frequency as therapy of diabetic macular oedema, exudative age-related macular degeneration and other intraocular neovascular or oedematous diseases.1, 2 According to initial pilot studies dating back up to 4 years, the list of reported complications includes infectious and noninfectious endophthalmitis, secondary ocular hypertension potentially leading to secondary steroid-induced open-angle glaucoma, and iatrogenic lesions to the intraocular structures.2, 3, 4, 5 Despite its wide-spread use, studies on large numbers of injections have mostly been missing, yet. It was, therefore, the purpose of the present study to report on the injection-related complications, which were observed after intravitreal applications of triamcinolone and bevacizumab within an 8-year period in a single center. Since previous studies have dealt in detail with the frequency and amount of steroid-induced elevation of intraocular pressure,5 that specific complication of intravitreal triamcinolone injection was not addressed in the present investigation.
The clinical retrospective study included all 5403 intravitreal injections of about 20 mg triamcinolone acetonide (n=1588) or 1.5 mg bevacizumab (n=3818) consecutively performed in the period from 2000 to 2007 by three surgeons (UHS, FS, JBJ) for therapy of various intraocular oedematous or neovascular diseases including age-related macular degeneration and diabetic macular oedema. Follow-up after each injection was at least 3 months. All patients who received the injections in the department of ophthalmology, were followed up either by the department itself, or by the referring ophthalmologists, who lived at a distance of maximal 50 km. According to the cooperation between the referring ophthalmologists and the department, patients with injection-related complications such as endophthalmitis, cataract or retinal detachment were re-referred to the department on an emergency basis. The study was a retrospective analysis of patients who received a standard treatment. The patients were fully informed about the experimental character of the treatment and signed an informed consent. An approval of the ethics committee was obtained for the triamcinolone injections. All injections were carried under sterile conditions in the operation theatre, as described previously.2
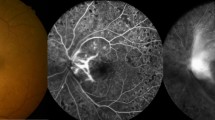
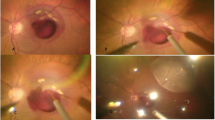
An infectious endophthalmitis, which necessitated a pars plana vitrectomy was detected in two eyes (2/5403 or 0.04%; 95% confidence interval (CI): 0.01, 0.09%) from the bevacizumab group at 2 days after the injection. Two eyes (2/5403 or 0.04%) from the bevacizumab group showed a painless vitreous clouding within 1 week after the injections. The clouding subsided after intensified topical antibiotic therapy. One eye (1/5403 or 0.02%) developed a retinal detachment about 2 months after the injection, and three eyes (3/5403 or 0.06±0.03%) (two eyes from the bevacizumab group) showed progressive cataracts which were operated on within 6 weeks after the injection. The total rate of these complications was 8/5403 or 0.15% (95%% CI: 0.05, 0.25%). The rate of complications was statistically independent of the surgeon (P=0.18), the drug injected (P=0.45), and the age of the patients (P=0.87).
In conclusion, during a follow-up of 3 months, injection-related complications such as infectious endophthalmitis, retinal detachment, and traumatic cataract may occur with a relatively low frequency of about 0.15±0.05% after intravitreal injections of bevacizumab or triamcinolone, independently of the drug injected, and the age of the patients. These figures, which do not relate to drug-specific complications such as steroid-induced ocular hypertension and steroid-induced cataract, are similar to those reported in previous investigations on smaller sample sizes or in multicentric studies. These injection-related risks compare favourably with the therapeutic benefit by the intravitreal therapy.1, 2, 4
References
Rosenfeld PJ, Moshfeghi AA, Puliafito CA . Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005; 36: 331–335.
Jonas JB, Kreissig I, Söfker A, Degenring RF . Intravitreal injection of triamcinolone acetonide for diabetic macular edema. Arch Ophthalmol 2003; 121: 57–61.
Jonas JB, Kreissig I, Degenring RF . Endophthalmitis after intravitreal injection of triamcinolone acetonide. Arch Ophthalmol 2003; 121: 1663–1664.
Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME . Twelve-month safety of intravitreal injections of bevacizumab (Avastin(R)): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol 2008; 246: 81–87.
Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA . Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology 2005; 112: 593–598.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proprietary interest: None
Rights and permissions
About this article
Cite this article
Jonas, J., Spandau, U. & Schlichtenbrede, F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye 22, 590–591 (2008). https://doi.org/10.1038/eye.2008.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.10
Keywords
This article is cited by
-
Katarakt durch iatrogenes Linsentrauma im Rahmen einer intravitrealen Injektion – Darstellung mittels (intraoperativer) optischer Kohärenztomographie
Die Ophthalmologie (2024)
-
Nanotechnology-based ocular drug delivery systems: recent advances and future prospects
Journal of Nanobiotechnology (2023)
-
Liposomes for effective drug delivery to the ocular posterior chamber
Journal of Nanobiotechnology (2019)
-
Rate of intraoperative complications during cataract surgery following intravitreal injections
Eye (2016)
-
Management of iatrogenic crystalline lens injury occurred during intravitreal injection
International Ophthalmology (2016)