To the Editor—Outpatient parenteral antimicrobial therapy (OPAT) is a safe, clinically effective, convenient and cost-effective way of administering parenteral antimicrobials while allowing the patient to reside in the community.Reference Tice, Rehm and Dalovisio1, Reference Gordon, Shrestha and Rehm2 It has been hypothesized that OPAT reduces the incidence of hospital-acquired infections by cutting down the length of stay and exposure to the healthcare facility.Reference Chapman, Dixon, Andrews, Lillie, Bazaz and Patchett3 However, there are very few studies on the incidence and outcomes of OPAT in relation to C. difficile infection (CDI). Two previous European studies have reported <1% incidence of CDI in their OPAT population.Reference Chapman, Dixon, Andrews, Lillie, Bazaz and Patchett3, Reference Barr, Semple and Seaton4 A recent questionnaire survey of infectious diseases (ID) physicians conducted by the Infectious Diseases Society of America (IDSA), reported that the incidence of CDI in the OPAT population may be higher than that reported in the European studies.Reference Lane, Marschall and Beekmann5 With increasing reports of community onset/acquired CDI with minimal or no exposure to a healthcare facility,Reference Kutty, Woods and Sena6 it is possible that OPAT patients on broad-spectrum antibiotics are at increased risk of CDI.
Our institution has a large and well-organized OPAT program in which all antimicrobial management is supervised by infectious disease staff physicians, and infectious disease follow-up arrangements are made before patients are discharged from hospital.Reference Gordon, Shrestha and Rehm2 This arrangement provided the opportunity to examine the incidence and outcomes of CDI in our OPAT cohort. The study protocol was approved by the Institutional Review Board of Cleveland Clinic. We retrospectively reviewed all adult patients discharged home on OPAT from January 1, 2013, to January 1, 2014, whose OPAT medications were provided by the Cleveland Clinic Home Infusion Pharmacy (CCHIP). Community-onset, healthcare facility–associated (CO-HCFA) CDI was defined as diarrheal symptoms (watery diarrhea>3 times per day) and positive stool toxin-B polymerase chain reaction (PCR) for Clostridium difficile, in the community or within the first 3 days of readmission, provided the diagnosis was made less than 4 weeks since discharge from a healthcare facility. CDI severity was defined per the Infectious Disease Society of America (IDSA)Reference Cohen, Gerding and Johnson7; recurrence was defined as symptomatic diarrhea with positive-stool PCR within 8 weeks of successful treatment of the previous episode. In patients with multiple OPAT courses, only the initial OPAT course was included.
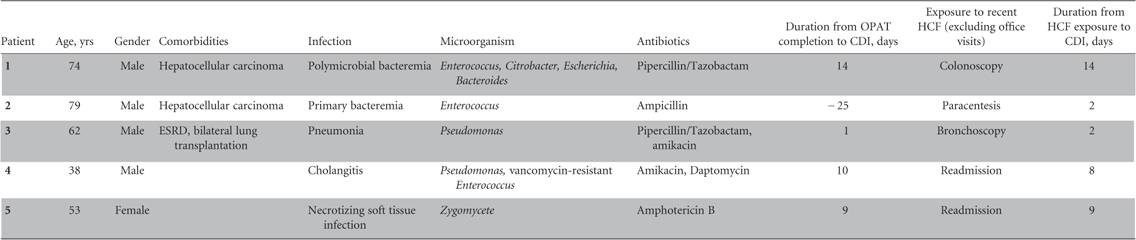
During the study period, a total of 1,462 patients received one or more courses of OPAT at home. Of these, 681 patients received medications for at least 1 OPAT course via the Cleveland Clinic Home Infusion Pharmacy. Five of these 681 patients (0.73%) developed CO-HCFA CDI with an incidence rate of 0.26 cases per 1000 patient-days. Table 1 summarizes the demographic characteristics of and outcomes for these 5 patients. The most frequently administered antimicrobials were pipercillin/tazobactam and amikacin, with a median duration of 12 days (interquartile range [IQR]: 6.5–28). Four of these 5 patients completed OPAT prior to developing CO-HCFA CDI. The median period between OPAT completion to developing CDI was 9.5 days (IQR: 3–13). Prior to developing CDI, each of the 5 patients had direct exposure to a healthcare facility that was more than a simple follow-up office visit after discharge from the hospital. The median duration from healthcare facility exposure to development of CDI was 8 days (IQR: 2–11.5). Of these 5 patients, 2 had hospital readmissions (non–CDI related) and 3 had outpatient procedures. Four of these 5 patients (80%) were on concomitant acid-suppressive therapy. All patients had mild-to-moderate CDI and responded well to medical therapy. None of the 5 patients had a recurrent CDI, and there was no attributable 90-day mortality.
TABLE 1 Demographic Characteristics and Outcomes of Patients with Clostridium difficile Infection (CDI) Discharged with Outpatient Parenteral Antimicrobial Therapy (OPAT)

OPAT=outpatient parenteral antimicroboial therapy; ESRD=end-stage renal disease; HCF=healthcare facility.
In this large study of 681 OPAT patients, CO-HCFA CDI was uncommon, occurring in <1% of patients and at an incidence rate of less than 1 per 1000 person-days. None of the patients developed severe CDI; CDI did not progress to recurrent CDI; and there were no CDI-related readmissions. All CO-HCFA CDI patients had an institutional healthcare exposure, and 80% of people who developed CDI were on concomitant acid-suppression therapy.
Our findings on the incidence of CDI are in agreement with that reported in the two European studies.Reference Chapman, Dixon, Andrews, Lillie, Bazaz and Patchett3, Reference Barr, Semple and Seaton4 The two major risk factors for developing CDI are antimicrobial therapy and healthcare facility exposure.Reference Cohen, Gerding and Johnson7 Four of the 5 patients in our study developed CDI with 10 days of completing their antibiotic OPAT medications and all five patients had a recent healthcare facility exposure other than their regular follow up office visits, after they were discharged from hospital and before the development of CDI. Both inpatient and outpatient healthcare exposure have been attributed to increased risk of CDI development.Reference Samore, DeGirolami, Tlucko, Lichtenberg, Melvin and Karchmer8, Reference Jury, Sitzlar and Kundrapu9 Therefore, antimicrobial stewardship and effective infection control measures will play an important role in further reducing the incidence of CDI in this population. Four of the 5 patients in our study were on concomitant acid-suppressive therapy; a few recent meta-analyses have suggested an association between proton-pump inhibitor (PPI) exposure and risk of developing CDI.Reference Deshpande, Pant and Pasupuleti10 This underscores the need for more judicious use of this class of medications.
Our study has some inherent limitations. The major limitation is that it is based on retrospective observational data. CDIs that were diagnosed based on tests done outside the Cleveland Clinic Health System were not identified. The small number of CDIs precludes an analysis for risk factors associated with development of CDIs in OPAT patients. Finally, our study is a single-center experience, and additional studies are needed in other settings.
Overall, this study showed that CO-HCFA CDI is uncommon in the OPAT population. This helps physicians weigh the risks and benefits of OPAT and emphasizes the need for continued follow-up beyond the termination of antibiotic therapy.
Acknowledgments
Financial support. None reported.
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.