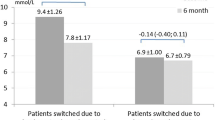
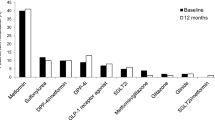
Abstract
Insulin glargine 300 U/mL (Toujeo®) is a long-acting basal insulin analogue approved for the treatment of diabetes mellitus. Insulin glargine 300 U/mL has a more stable and prolonged pharmacokinetic/pharmacodynamic profile than insulin glargine 100 U/mL (Lantus®), with a duration of glucose-lowering activity exceeding 24 h. In several 6-month phase III trials, insulin glargine 300 U/mL achieved comparable glycaemic control to that seen with insulin glargine 100 U/mL in patients with type 1 or type 2 diabetes, albeit with consistently higher daily basal insulin requirements. These improvements in glycaemic control were maintained during longer-term (12 months) treatment. Insulin glargine 300 U/mL was generally associated with a lower risk of nocturnal hypoglycaemia than insulin glargine 100 U/mL in insulin-experienced patients with type 2 diabetes, while the risk of nocturnal hypoglycaemia did not significantly differ between treatment groups in insulin-naïve patients with type 2 diabetes or in patients with type 1 diabetes. To conclude, once-daily subcutaneous insulin glargine 300 U/mL is an effective and generally well tolerated basal insulin therapy option for patients with type 1 or type 2 diabetes.
Similar content being viewed by others
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
American Diabetes Association. Standards of medical care in diabetes—2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97–111.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: what progress have we made? Diabetes Metab Res Rev. 2014;30(2):104–19.
Maiorino MI, Petrizzo M, Capuano A, et al. The development of new basal insulins: is there any clinical advantage with their use in type 2 diabetes? Expert Opin Biol Ther. 2014;14(6):799–808.
Woo VC. New insulins and new aspects in insulin delivery. Can J Diabetes. 2015;39(4):335–43.
Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73(6):575–93.
Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 Units. mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units. mL-1. Diabetes Care. 2015;38(4):637–43.
Shiramoto M, Eto T, Irie S, et al. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17(3):254–60.
Dunn CJ, Plosker GL, Keating GM, et al. Insulin glargine: an updated review of its use in the management of diabetes mellitus. Drugs. 2003;63(16):1743–78.
European Medicines Agency. Toujeo (insulin glargine U300): summary of product characteristics. 2015. http://www.ema.europa.eu/. Accessed 19 Jan 2016.
Becker RHA, Nowotny I, Teichert L, et al. Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab. 2015;17(3):261–7.
Bergenstal R, Bailey TS, Rodbard D, et al. Insulin glargine 300 U/mL vs 100 U/mL: glucose profiles of morning vs evening injections in adults with T1DM measured with continuous glucose monitoring (CGM) [abstract no. 39]. Diabetes Technol Ther. 2015;17(Suppl 1):A16–7.
Jinnouchi H, Koyama M, Amano A, et al. Continuous glucose monitoring during basal-bolus therapy using insulin glargine 300 U mL-1 and glargine 100 U mL-1 in Japanese people with type 1 diabetes mellitus: a crossover pilot study. Diabetes Ther. 2015;6(2):143–52.
Sanofi-Aventis US LLC. Prescribing information for Toujeo (insulin glargine injection) U-300. 2015. http://www.accessdata.fda.gov. Accessed 19 Jan 2016.
Steinstraesser A, Schmidt R, Bergmann K, et al. Investigational new insulin glargine 300 U/ml has the same metabolism as insulin glargine 100 U/ml. Diabetes Obes Metab. 2014;16(9):873–6.
European Medicines Agency. Assessment report: Toujeo (insulin glargine). 2015. http://www.ema.europa.eu/. Accessed 19 Jan 2016.
Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 Units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37(10):2755–62.
Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 Units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37(12):3235–43.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386–94.
Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 Units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015;38(12):2217–25.
Matsuhisa M, Koyama M, Cheng X, et al. New insulin glargine 300 U/mL: glycemic control and hypoglycemia in Japanese people with T1DM (EDITION JP 1) [abstract no. 88-LB]. Diabetes. 2014;63(Suppl 1A):LB22.
Terauchi Y, Koyama M, Cheng X, et al. Glycemic control and hypoglycemia in Japanese people with T2DM receiving new insulin glargine 300 U/mL in combination with OADs (EDITION JP 2) [abstract no. 94-LB]. Diabetes. 2014;63(Suppl 1A):LB24.
Sanofi. Comparison of a new formulation of insulin glargine with Lantus® in Japanese patients with type 1 diabetes mellitus (EDITION JP I). 2012. https://www.clinicaltrials.gov/ct2/show/NCT01689129. Accessed 19 Jan 2016.
Sanofi. Comparison of a new formulation of insulin glargine with Lantus® both in combination with oral antihyperglycemic drug(s) in Japanese patients with type 2 diabetes mellitus (EDITION JP II). 2012. https://www.clinicaltrials.gov/ct2/show/NCT01689142. Accessed 19 Jan 2016.
Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17(9):859–67.
Buzzetti R, Pettus JH, Brito-Sanfiel M, et al. New insulin glargine 300 U/mL (Gla-300) in combination with dipeptidyl peptidase IV inhibitors in T2DM (EDITION 2 and 3): glycemic control and hypoglycemia [abstract no. 95-LB]. Diabetes. 2015;64(Suppl A1):LB24.
Twigg SM, Escalada J, Grisoni ML, et al. Age, BMI, and diabetes duration: effect on glycemic control and hypoglycemia with insulin glargine 300 U/mL in type 2 diabetes (T2DM) [abstract no. 1017-P]. Diabetes. 2015;64(Suppl 1):A260.
Yale JF, Aroda VR, Charbonnel B, et al. Older people with type 2 diabetes: glycemic control and hypoglycemia risk with new insulin glargine 300 U/mL [abstract no. 991-P]. Diabetes. 2015;64(Suppl 1):A252.
Roussel R, D’Emden MC, Fisher M, et al. Switching from twice-daily basal insulin to once-daily new insulin glargine 300 U/mL (GLA-300): an analysis in people with T2DM (EDITION 1 and 2) [abstract no. 1021-P]. Diabetes. 2015;64(Suppl 1):A261.
Riddle MC, Bolli GB, Home PD, et al. New insulin glargine 300 U/mL: efficacy and safety of flexible vs fixed dosing intervals in people with type 2 diabetes mellitus [abstract no. 234]. Diabetes Technol Ther. 2015;17(Suppl 1):A102–3.
Riddle MC, Yki-Jarvinen H, Bolli GB, et al. One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension. Diabetes Obes Metab. 2015;17(9):835–42.
Yki-Jarvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs (EDITION 2 randomised 12-month trial including 6-month extension). Diabetes Obes Metab. 2015;17(12):1142–9.
Ritzel RA, Roussel R, Giaccari A, et al. Patient-level meta-analysis of 1y phase 3a EDITION type 2 diabetes mellitus studies: glycaemic control and hypoglycaemia with insulin glargine 300 U/ml (Gla-300) vs glargine 100 U/ml (Gla-100) [abstract no. 975]. Diabetologia. 2015;58(Suppl 1):S472.
Matsuhisa M, Koyama M, Cheng X, et al. Sustained glycemic control and less nocturnal hypoglycemia with new insulin glargine 300 U/mL compared with glargine 100 U/mL over 12 months in Japanese people with T1DM (EDITION JP 1) [abstract no. 987-P]. Diabetes. 2015;64(Supp l):A250.
Terauchi Y, Koyama M, Cheng X, et al. New insulin glargine 300 U/mL provides sustained glycemic control and reduced hypoglycemia over 12 months compared with glargine 100 U/mL in Japanese people with T2DM managed with basal insulin plus OAD(s) (EDITION JP 2) [abstract no. 98-OR]. Diabetes. 2015;64(Suppl 1):A26.
Riddle MC, Home PD, Avogaro A, et al. A clinically-defined nocturnal window for analysis of hypoglycemia with new insulin glargine 300 U/mL in type 2 diabetes (T2DM) [abstract no. 1027-P]. Diabetes. 2015;64(Suppl 1):A263.
Rosenstock J, Zhang Q, Gerrits C, et al. Is hypoglycemia a modifiable patient risk in type 2 diabetes? A pooled analysis of insulin glargine 300U/ml (Gla-300) vs. 100U/ml (Gla-100) trials [abstract no. 423-P]. Diabetes. 2015;64(Suppl 1):A110.
Eli Lilly. Lilly ends basal insulin peglispro development program. 2015. https://investor.lilly.com. Accessed 19 Jan 2016.
Becker RH, Frick AD, Teichert L, et al. Fluctuation and reproducibility of exposure and effect of insulin glargine in healthy subjects. Diabetes Obes Metab. 2008;10(11):1105–13.
Pohlmeier H, Klonoff DC, Berard L, et al. Ease of use of the new insulin glargine 300 U/ml solostar pen injector in insulin-naive people with type 2 diabetes [abstract no. 1052-P]. Diabetes. 2015;64(Suppl 1):A269–70.
Klonoff D, Nayberg I, Erbstein F, et al. Usability of the Gla-300 injection device compared with three other commercialized disposable insulin pens: results of an interview-based survey. J Diabetes Sci Technol. 2015;9(4):936–8.
Sanofi. A “real world” trial to determine efficacy and health outcomes of Toujeo (achieve control real life study program). 2015. https://www.clinicaltrials.gov/ct2/show/NCT02451137. Accessed 19 Jan 2016.
Acknowledgments
During the peer review process, the manufacturer of insulin glargine 300 U/mL was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Hannah Blair and Gillian Keating are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: J. G. Eriksson, Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; A. B. King, Diabetes Care Center, Salinas, CA, USA; A. J. Scheen, Division of Diabetes, Nutrition and Metabolic Disorders, University of Liege, Liege, Belgium; Y. Terauchi, Department of Endocrinology and Metabolism, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Rights and permissions
About this article
Cite this article
Blair, H.A., Keating, G.M. Insulin Glargine 300 U/mL: A Review in Diabetes Mellitus. Drugs 76, 363–374 (2016). https://doi.org/10.1007/s40265-016-0541-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0541-z