Abstract
Purpose of Review
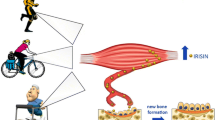
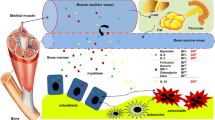
Skeletal muscle and bone are connected anatomically and physiologically, and play a crucial role in human locomotion and metabolism. Historically, the coupling between muscle and bone has been viewed in light of mechanotransduction, which dictates that the mechanical forces applied to muscle are transmitted to the skeleton to initiate bone formation. However, these organs also communicate through the endocrine system, orchestrated by a family of cytokines namely myokines (derived from myocytes) and osteokines (derived from bone cells). A third player in this biochemical crosstalk is adipose tissue and the secretion of adipokines (derived from adipocytes). In this review, we discuss the bidirectional effects of myokines and osteokines on muscle and bone metabolism, and the impact of adipokines on both of these secretory organs.
Recent Findings
Several myokines, notably, IL6, irisin, IGF-1, BDNF, myostatin, and FGF2 exert anabolic/catabolic effects on bone, while the osteokines osteocalcin and sclerostin have shown to induce muscle anabolism and catabolism, respectively. Adipokines, such as leptin, resistin, adiponectin, and TNFα (released from adipose tissue), can also modulate muscle and bone metabolism. Contrarily, exercise-mediated release of lipolytic myokines (IL6, irisin, and LIF) stimulates thermogenesis by promoting the browning of adipocytes.
Summary
Myokines, osteokines, and adipokines exert autocrine/paracrine effects locally as well as through the endocrine system, to regulate muscle, bone, and fat metabolism. Reductions in physical activity and increases in energy intake, both linked with aging, leads to adipocyte hypertrophy and the recruitment of immunological cells (macrophages). In turn, this releases pro-inflammatory adipokines which induces chronic low-grade inflammation (LGI), a key player in the pathology of several diseases. However, exercise-induced stimulation of bioactive cytokines, through muscle-bone-fat crosstalk, increases muscle anabolism, bone formation, mitochondrial biogenesis, glucose utilization, and fatty acid oxidation, and attenuates chronic LGI.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. Sichuan University. 2017:1–33.
Tieland M, Trouwborst I, Clark B. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle [Internet]. 2017;9:3–19 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5803609/.
Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr [Internet]. 2006 [cited 2020 Mar 5];84:475–482. Available from: https://pubmed.ncbi.nlm.nih.gov/16960159-the-underappreciated-role-of-muscle-in-health-and-disease/
Lombardi G. Exercise-dependent modulation of bone metabolism and bone endocrine function: new findings and therapeutic perspectives. J Sci Sport Exerc. Springer Science and Business Media LLC; 2019;1:20–28.
Curtis E, Litwic A, Cooper C, Dennison E. Determinants of muscle and bone aging. J Cell Physiol. 2015;230:2618–25.
Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone [Internet]. 1st ed. Adv. Clin. Chem. Elsevier Inc.; 2020. Available from: https://doi.org/10.1016/bs.acc.2019.07.010
Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. This paper is one of a selection of papers published in this Special Issue, entitled 14th International Biochemistry of Exercise Conference – Muscles as Molecular and Metabolic Machines, and has undergone the Journal’s usual peer review process. Appl Physiol Nutr Metab [Internet]. 2009 [cited 2018 Sep 14];34:377–81. Available from: https://doi.org/10.1139/H09-012
Chen H, Zhou X, Fujita H, Onozuka M, Kubo K-Y. Age-related changes in trabecular and cortical bone microstructure [Internet]. Int. J. Endocrinol. Hindawi Publishing Corporation; 2013. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614119/
Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC Geriatr [Internet]. BioMed Central; 2013 [cited 2018 Sep 13];13:71. Available from: https://doi.org/10.1186/1471-2318-13-71
Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol [Internet]. 2000 [cited 2018 Sep 4];89:81–8. Available from: http://www.jap.org
Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. Vina J, editor. PLoS One [Internet]. Public Library of Science; 2014 [cited 2018 Nov 8];9:e113637. Available from: https://doi.org/10.1371/journal.pone.0113637
Elphingstone J, Hamrick MW. Muscle and bone biology – similarities and differences. Osteosarcopenia Bone, Muscle Fat Interact. Springer International Publishing; 2019. p. 3–27.
Laurent MR, Dedeyne L, Dupont J, Mellaerts B, Dejaeger M, Gielen E. Age-related bone loss and sarcopenia in men. Maturitas [Internet]. Elsevier; 2019 [cited 2019 Aug 29];122:51–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30797530.
Dolan E, Sale C. Protein and bone health across the lifespan. Proc Nutr Soc. 2019;78:45–55.
De Rui M, Inelmen EM, Pigozzo S, Trevisan C, Manzato E, Sergi G. Dietary strategies for mitigating osteosarcopenia in older adults: a narrative review. Aging Clin Exp Res. 2019.
Yang A, Lv Q, Chen F, Wang Y, Liu Y, Shi W, et al. The effect of vitamin D on sarcopenia depends on the level of physical activity in older adults. J Cachexia Sarcopenia Muscle [Internet]. Wiley Blackwell; 2020 [cited 2020 Mar 5];jcsm.12545. Available from: https://doi.org/10.1002/jcsm.12545
McGlory C, Phillips SM. Exercise and the regulation of skeletal muscle hypertrophy. Prog Mol Biol Transl Sci. 2015;135:153–73.
Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. John Wiley & Sons, Ltd (10.1111). 2013;591:2911–23.
Wallace TC, Frankenfeld CL. Dietary protein intake above the current RDA and bone health: a systematic review and meta-analysis. J Am Coll Nutr Routledge. 2017:481–96.
Verlaan S, Maier AB, Bauer JM, Bautmans I, Brandt K, Donini LM, et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults - The PROVIDE study. Clin Nutr [Internet]. Elsevier Ltd; 2016 [cited 2019 Feb 11];37:1–7. Available from: https://www.sciencedirect.com/science/article/pii/S0261561417300109
Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin d on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab Endocrine Society. 2014:4336–45.
Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, et al. Protein intake and mobility limitation in community-dwelling older adults: the health ABC study. J Am Geriatr Soc. 2017;65:1705–11.
Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB. Dietary protein intake is associated with lean mass change in older , community-dwelling adults : the Health , aging , and body composition ( Health ABC ) study. Am J Clin Nutr. 2008;87:150–5.
Medina-Gomez C, Kemp JP, Dimou NL, Kreiner E, Chesi A, Zemel BS, et al. Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus. Nat Commun [Internet]. Springer US; 2017 [cited 2019 Jul 17];8:1–10. Available from: https://doi.org/10.1038/s41467-017-00108-3
Trajanoska K, Rivadeneira F. Genetics of osteosarcopenia. Osteosarcopenia bone, muscle fat interact [Internet]. Cham: Springer Nature Switzerland AG; 2019 [cited 2019 Nov 26]. p. 215–30. Available from: https://doi.org/10.1007/978-3-030-25890-0_10
Kirk B, Al Saedi A, Duque G. Osteosarcopenia: a case of geroscience. aging med [Internet]. 2019;00:1–10. Available from: https://doi.org/10.1002/agm2.12080, 2019.
Wong RMY, Wong H, Zhang N, Chow SKH, Chau WW, Wang J, et al. The relationship between sarcopenia and fragility fracture—a systematic review. Osteoporos Int [Internet]. Springer London; 2019 [cited 2019 May 29];30:541–53. Available from: https://doi.org/10.1007/s00198-018-04828-0
McGlory C, von Allmen MT, Stokes T, Morton RW, Hector AJ, Lago BA, et al. Failed recovery of glycemic control and myofibrillar protein synthesis with 2 wk of physical inactivity in overweight, prediabetic older adults. Journals Gerontol Ser A [Internet]. Oxford University Press; 2018 [cited 2018 Aug 6];73:1070–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29095970.
Ren R, Ocampo A, Liu GH, Izpisua Belmonte JC. Regulation of stem cell aging by metabolism and epigenetics. Cell Metab. Cell Press. 2017:460–74.
Al Saedi A, Goodman CA, Myers DE, Hayes A, Duque G. Rapamycin affects palmitate-induced lipotoxicity in osteoblasts by modulating apoptosis and autophagy. Journals Gerontol Ser A [Internet]. 2019 [cited 2019 Jul 15]; Available from: https://doi.org/10.1093/gerona/glz149/5520593
Al Saedi A, Bermeo S, Plotkin L, Myers DE, Duque G. Mechanisms of palmitate-induced lipotoxicity in osteocytes. Bone. 2019.
Al Saedi A, Hassan EB, Duque G. The diagnostic role of fat in osteosarcopenia. J Lab Precis Med [Internet]. 2019;4:7–7 Available from: http://jlpm.amegroups.com/article/view/4911/html.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest American Society for Clinical Investigation; 2011. p. 2111–2117, 121.
Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43–7.
Brotto M, Bonewald L. Bone and muscle: interactions beyond mechanical. Bone [Internet]. 2015 [cited 2019 Oct 1];80:109–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26453500.
•• Chowdhury S, Schulz LC, Palmisano B, Singh P, Berger JM, Yadav VK, et al. Muscle derived interleukin-6 increases exercise capacity by signaling in osteoblasts. J Clin Invest. American Society for Clinical Investigation. 2020; Uncovered a novel mechanism of bone-muscle cross talk surrounding OCN and IL6 signaling.
Lombardi G, Sanchis-Gomar F, Perego S, Sansoni V, Banfi G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine. 2016.
Lombardi G, Perego S, Luzi L, Banfi G. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine. Humana Press Inc. 2015:394–404.
Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. The Rockefeller University Press. 1995;182:1461–8.
Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. Academic Press. 2007:75–83.
Perera PY, Lichy JH, Waldmann TA, Perera LP. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect. Elsevier Masson. 2012:247–61.
Kaji H. Effects of myokines on bone. Bonekey Rep Portico. 2016;5.
• Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem. American Society for Biochemistry and Molecular Biology Inc. 2017;292:11021–33 Identified possible mechanisms of the interaction between myostatin and bone.
Wallner C, Jaurich H, Wagner JM, Becerikli M, Harati K, Dadras M, et al. Inhibition of GDF8 (Myostatin) accelerates bone regeneration in diabetes mellitus type 2. Sci Rep Nature Publishing Group. 2017;7.
Sun L, Sun S, Zhao X, Zhang J, Guo J, Tang L, et al. Inhibition of myostatin signal pathway may be involved in low-intensity pulsed ultrasound promoting bone healing. J Med Ultrason Springer Tokyo. 2019;46:377–88.
Hamrick MW. The skeletal muscle secretome: an emerging player in muscle–bone crosstalk. Bonekey Rep. Portico. 2012;1.
DiGirolamo DJ, Mukherjee A, Fulzele K, Gan Y, Cao X, Frank SJ, et al. Mode of growth hormone action in osteoblasts. J Biol Chem. American Society for Biochemistry and Molecular Biology. 2007;282:31666–74.
Steringer JP, Müller HM, Nickel W. Unconventional secretion of fibroblast growth factor 2 - a novel type of protein translocation across membranes? J. Mol. Biol. Academic Press. 2015:1202–10.
Adhikary S, Choudhary D, Tripathi AK, Karvande A, Ahmad N, Kothari P, et al. FGF-2 targets sclerostin in bone and myostatin in skeletal muscle to mitigate the deleterious effects of glucocorticoid on musculoskeletal degradation. Life Sci. Elsevier Inc. 2019;229:261–76.
Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci U S A. National Academy of Sciences. 2012;109:3143–8.
Li X, Stanislaus S, Asuncion F, Niu QT, Chinookoswong N, Villasenor K, et al. FGF21 is not a major mediator for bone homeostasis or metabolic actions of PPARα and PPARγ agonists. J Bone Miner Res [Internet]. John Wiley and Sons Inc; 2017 [cited 2020 Mar 6];32:834–45. Available from: https://doi.org/10.1002/jbmr.2936
Arhire LI, Mihalache L, Covasa M. Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne). Frontiers Media SA. 2019;10:524.
Liu JJ, Wong MDS, Toy WC, Tan CSH, Liu S, Ng XW, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. Elsevier. 2013;27:365–9.
Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. Springer-Verlag London Ltd. 2014;25:1633–42.
Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G, et al. Irisin enhances osteoblast differentiation in vitro. 2014 [cited 2020 Mar 6]; Available from: https://doi.org/10.1155/2014/902186
•• Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. National Academy of Sciences. 2015;112:12157–62 Presents mechanistic evidence for the anabolic impact of irisin of bone.
Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. Sichuan University; 2017;5:1–14.
Qiao XY, Nie Y, Ma YX, Chen Y, Cheng R, Yinrg WY, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. Nature Publishing Group; 2016;6:1–12.
Leal LG, Lopes MA, Batista ML. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. Frontiers Media S.A. 2018.
Kraemer RR, Shockett P, Webb ND, Shah U, Castracane VD. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm Metab Res [Internet]. © Georg Thieme Verlag KG; 2014 [cited 2020 Mar 6];46:150–4. Available from: https://doi.org/10.1055/s-0033-1355381
Aydin S, Aydin S, Kuloglu T, Yilmaz M, Kalayci M, Sahin I, et al. Alterations of irisin concentrations in saliva and serum of obese and normal-weight subjects, before and after 45 min of a Turkish bath or running. Peptides. Elsevier. 2013;50:13–8.
Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol [Internet]. John Wiley & Sons, Ltd; 2013 [cited 2020 Mar 6];591:5393–400. Available from: https://doi.org/10.1113/jphysiol.2013.263707
• Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell [Internet]. 2007 [cited 2019 Oct 1];130:456–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17693256. First study identifying the endocrine role of osteocalcin on metabolism.
Lin CF, Huang TH, Tu KC, Lin LL, Tu YH, Yang R Sen. Acute effects of plyometric jumping and intermittent running on serum bone markers in young males. Eur J Appl Physiol. Springer; 2012;112:1475–1484.
Ahn N, Kim K. Effects of 12-week exercise training on osteocalcin, high-sensitivity C-reactive protein concentrations, and insulin resistance in elderly females with osteoporosis. J Phys Ther Sci [Internet]. Society of Physical Therapy Science (Rigaku Ryoho Kagakugakkai); 2016 [cited 2020 Mar 6];28:2227–31. Available from: https://www.jstage.jst.go.jp/article/jpts/28/8/28_jpts-2016-176/_article
Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, Ahn CW. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin Endocrinol (Oxf) [Internet]. Blackwell Publishing Ltd; 2015 [cited 2020 Mar 6];82:686–94. Available from: https://doi.org/10.1111/cen.12601
Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. Cell Press. 2016;23:1078–92.
Liu S, Gao F, Wen L, Ouyang M, Wang Y, Wang Q, et al. Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 myoblast cells. Cell Physiol Biochem [Internet]. S. Karger AG; 2017 [cited 2020 Mar 6];43:1100–12. Available from: https://www.karger.com/Article/FullText/481752
Pi M, Kapoor K, Ye R, Nishimoto SK, Smith JC, Baudry J, et al. Evidence for osteocalcin binding and activation of GPRC6A in β-cells. Endocrinology [Internet]. Endocrine Society; 2016 [cited 2020 Mar 6];157:1866–80. Available from: https://doi.org/10.1210/en.2015-2010
Lin X, Parker L, McLennan E, Hayes A, McConell G, Brennan-Speranza TC, et al. Undercarboxylated osteocalcin improves insulin-stimulated glucose uptake in muscles of corticosterone-treated mice. J Bone Miner Res [Internet]. John Wiley and Sons Inc.; 2019 [cited 2020 Mar 6];34:1517–30. Available from: https://doi.org/10.1002/jbmr.3731
Lin X, Parker L, Mclennan E, Zhang X, Hayes A, McConell G, et al. Recombinant uncarboxylated osteocalcin per se enhances mouse skeletal muscle glucose uptake in both extensor digitorum longus and soleus muscles. Front Endocrinol (Lausanne) [Internet]. Frontiers Media S.A.; 2017 [cited 2020 Mar 6];8:330. Available from: https://doi.org/10.3389/fendo.2017.00330/full
Levinger I, Lin X, Zhang X, Brennan-Speranza TC, Volpato B, Hayes A, et al. The effects of muscle contraction and recombinant osteocalcin on insulin sensitivity ex vivo. Osteoporos Int. Springer-Verlag London Ltd; 2016;27:653–663.
Lin X, Hanson E, Betik AC, Brennan-Speranza TC, Hayes A, Levinger I. Hindlimb immobilization, but not castration, induces reduction of undercarboxylated osteocalcin associated with muscle atrophy in rats. J Bone Miner Res [Internet]. John Wiley and Sons Inc.; 2016 [cited 2020 Mar 6];31:1967–78. Available from: https://doi.org/10.1002/jbmr.2884
Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. Elsevier GmbH. 2016;5:1042–7.
Mohammad Rahimi GR, Bijeh N, Rashidlamir A. Effects of exercise training on serum preptin, undercarboxylated osteocalcin and high molecular weight adiponectin in adults with metabolic syndrome. Exp Physiol [Internet]. Blackwell Publishing Ltd; 2019 [cited 2020 Mar 6];105:449–59. Available from: https://doi.org/10.1113/EP088036
Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, et al. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. Elsevier Inc. 2014;64:8–12.
Wood CL, Pajevic PD, Gooi JH. Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Reports. Elsevier Inc. 2017;6:74–80.
Karczewska-Kupczewska M, Stefanowicz M, Matulewicz N, Nikołajuk A, Strączkowski M. Wnt signaling genes in adipose tissue and skeletal muscle of humans with different degrees of insulin sensitivity. J Clin Endocrinol Metab [Internet]. Endocrine Society; 2016 [cited 2020 Mar 6];101:3079–87. Available from: https://doi.org/10.1210/jc.2016-1594
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus [Internet]. Wiley; 2017 [cited 2020 Mar 6];1:86–100. Available from: https://doi.org/10.1002/jbm4.10015
•• Kim JA, Roh E, Hyeon HS, Bin LY, Kim NHNH, Yoo HJ, et al. Association of serum sclerostin levels with low skeletal muscle mass: the Korean Sarcopenic Obesity Study (KSOS). Bone. Elsevier Inc. 2019;128:115053 This study showed that serum sclerostin levels were negatively associated with sarcopenia.
Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, et al. Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. Phillips WD, editor. PLoS One [Internet]. Public Library of Science; 2018 [cited 2020 Mar 6];13:e0194440. Available from: https://doi.org/10.1371/journal.pone.0194440
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004:2548–56.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. Annual Reviews. 2010;72:219–46.
Nimmo MA, Leggate M, Viana JL, King JA. The effect of physical activity on mediators of inflammation. Diabetes, Obes. Metab. Blackwell Publishing Ltd. 2013:51–60.
Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. BioScientifica Ltd. 2014:113–27.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. Nature Publishing Group. 2018:513–37.
Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. Endocrinology. 2004;145:5589–97.
Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol - Endocrinol Metab. Am J Physiol Endocrinol Metab. 2006;290:E961–7.
•• Aguirre L, Napoli N, Waters D, Qualls C, Villareal DT, Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab. Endocrine Society. 2014;99:3290–7 This study demonstrated the pathophysiological link between adiposity and fracture risk.
Codoñer-Franch P, Alonso-Iglesias E. Resistin: Insulin resistance to malignancy. Clin Chim Acta Elsevier. 2015:46–54.
Marques EA, Mota J, Viana JL, Tuna D, Figueiredo P, Guimarães JT, et al. Response of bone mineral density, inflammatory cytokines, and biochemical bone markers to a 32-week combined loading exercise programme in older men and women. Arch Gerontol Geriatr. Arch Gerontol Geriatr. 2013;57:226–33.
Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. J Bone Miner Res. 2011;26:2851–9.
Jamurtas AZ, Theocharis V, Koukoulis G, Stakias N, Fatouros IG, Kouretas D, et al. The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur J Appl Physiol. Eur J Appl Physiol. 2006;97:122–6.
Frydelund-Larsen L, Akerstrom T, Nielsen S, Keller P, Keller C, Pedersen BK. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am J Physiol - Endocrinol Metab. Am J Physiol Endocrinol Metab. 2007:292.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol Frontiers Media S.A. 2017.
Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. BioMed Central Ltd. 2017;22.
Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med [Internet]. 2006 [cited 2018 Nov 25];119:526.e9-526.e17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16750969.
Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol - Endocrinol Metab. Am J Physiol Endocrinol Metab. 2012;303:E410–21.
Pérez-Baos S, Prieto-Potin I, Román-Blas JA, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G. Mediators and patterns of muscle loss in chronic systemic inflammation. Front Physiol Frontiers Media S.A. 2018.
Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. Osteoporosis International. 2017;28:2781–90.
Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, et al. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J. Cachexia. Sarcopenia Muscle. Wiley Online Library. 2014:183–92.
McLaughlin M, Jacobs I. Exercise is medicine, but does it interfere with medicine? Exerc Sport Sci. Rev. Lippincott Williams and Wilkins. 2017:127–35.
Lombardi G, Ziemann E, Banfi G. Physical activity and bone health: what is the role of immune system? A narrative review of the third way. Front. Endocrinol. (Lausanne). Frontiers Media S.A. 2019:60.
Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care [Internet]. 2012 [cited 2019 Jul 5];15:12–22. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00075197-201201000-00004
Sansoni V, Vernillo G, Perego S, Barbuti A, Merati G, Schena F, et al. Bone turnover response is linked to both acute and established metabolic changes in ultra-marathon runners. Endocrine. Humana Press Inc. 2017;56:196–204.
•• Xu J, Lombardi G, Jiao W, Banfi G. Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sport. Med. Springer International Publishing. 2016:1165–82 This study systematically reviewed the effects of different exercise programs in women lifelong.
Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol Nat Rev Rheumatol. 2010:50–9.
Cheung AM, Giangregorio L. Mechanical stimuli and bone health: what is the evidence? Curr Opin Rheumatol. Curr Opin Rheumatol. 2012;24:561–6.
Chen D, Xie R, Shu B, Landay AL, Wei C, Reiser J, et al. Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann N Y Acad Sci Blackwell Publishing Inc. 2019:48–60.
• Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. National Academy of Sciences. 2017;114:E11238–47 This study showed the extraskeletal metabolic effects of bone-derived sclerostin.
Li DJ, Fu H, Zhao T, Ni M, Shen FM. Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism. W.B. Saunders. 2016;65:747–56.
Brun J, Berthou F, Trajkovski M, Maechler P, Foti M, Bonnet N. Bone regulates browning and energy metabolism through mature osteoblast/osteocyte PPARg expression. Diabetes. American Diabetes Association Inc. 2017;66:2541–54.
Zeng J, Jiang Y, Xiang S, Chen B. Serum bone morphogenetic protein 7, insulin resistance, and insulin secretion in non-diabetic individuals. Diabetes Res Clin Pract. Diabetes Res Clin Pract. 2011;93.
Klein D, Álvarez-Cubela S, Lanzoni G, Vargas N, Prabakar KR, Boulina M, et al. BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes. American Diabetes Association Inc. 2015;64:4123–34.
Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. Nature. 2012;481:314–20.
• Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise (Cell Metabolism (2016) 23(6) (1078–1092)(S1550413116302224)(https://doi.org/10.1016/j.cmet.2016.05.004)). Cell Metab. Cell Press; 2017. p. 218. This study highlighted the osteocalcin-mediated bone-muscle crosstalk.
El-Kamshoushi AAM, Hassan EM, Hassaan PS. Evaluation of serum level of Osteocalcin hormone in male infertility. Andrologia. Blackwell Publishing Ltd. 2017;49.
Karsenty G, Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell. Endocrinol. Mol Cell Endocrinol. 2014;382:521–6.
•• Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. Nature Publishing Group. 2017;543:385–90 This study demonstrated the role of bone-derived lipocalin 2 in regulating appetite and energy metabolism.
Paton CM, Rogowski MP, Kozimor AL, Stevenson JL, Chang H, Cooper JA. Lipocalin-2 increases fat oxidation in vitro and is correlated with energy expenditure in normal weight but not obese women. Obesity. Obesity (Silver Spring). 2013;21.
Mei LD, Mosialou I, Min LJ. Bone: another potential target to treat, prevent and predict diabetes. Diabetes, Obes. Metab. Blackwell Publishing Ltd. 2018:1817–28.
Pedersen BK. The diseasome of physical inactivity - and the role of myokines in muscle-fat cross talk. J Physiol J Physiol. 2009:5559–68.
Le Goff B, Blanchard F, Berthelot JM, Heymann D, Maugars Y. Role for interleukin-6 in structural joint damage and systemic bone loss in rheumatoid arthritis. Jt. Bone Spine. Elsevier Masson SAS. 2010:201–5.
Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-κB ligand, osteoprotegerin, and receptor activator of NF-κB in mouse calvariae. J Immunol. The American Association of Immunologists. 2002;169:3353–62.
Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK, et al. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol. American Physiological Society. 2011;111:251–9.
Broholm C, Mortensen OH, Nielsen S, Akerstrom T, Zankari A, Dahl B, et al. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol. Wiley-Blackwell. 2008;586:2195–201.
Sims NA, Johnson RW. Leukemia inhibitory factor: A paracrine mediator of bone metabolism. Growth Factors Growth Factors. 2012;30:76–87.
Haugen F, Norheim F, Lian H, Wensaas AJ, Dueland S, Berg O, et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol - Cell Physiol. Am J Physiol Cell Physiol. 2010:298.
Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: A critical interdisciplinary perspective. Int J Med Sci. Int J Med Sci. 2012:825–32.
Görgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J. Exercise and regulation of adipokine and myokine production. Prog Mol Biol Transl Sci. Elsevier B.V. 2015:313–36.
Okabe I, Kikuchi T, Mogi M, Takeda H, Aino M, Kamiya Y, et al. IL-15 and RANKL play a synergistically important role in osteoclastogenesis. J Cell Biochem. Wiley-Liss Inc. 2017, 118:739–47.
Nam J, Perera P, Gordon R, Jeong YH, Blazek AD, Kim DG, et al. Follistatin-like 3 is a mediator of exercise-driven bone formation and strengthening. Bone. Elsevier Inc. 2015;78:62–70.
Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. Nature Publishing Group. 2015;21:1085–90.
Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sport. Scand J Med Sci Sports. 2013:23.
Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. Academic Press Inc. 2014;450:1089–94.
Matthews VB, Åström MB, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. Springer Verlag. 2009;52:1409–18.
Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. Med Sci Sports Exerc. 2007;39:728–34.
Goekint M, De Pauw K, Roelands B, Njemini R, Bautmans I, Mets T, et al. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol. Eur J Appl Physiol. 2010;110:285–93.
Griffin ÉW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly ÁM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav Physiol Behav. 2011;104:934–41.
Schmidt-Kassow M, Schädle S, Otterbein S, Thiel C, Doehring A, Lötsch J, et al. Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport Neuroreport. 2012;23:889–93.
Gmiat A, Micielska K, Kozłowska M, Flis DJ, Smaruj M, Kujach S, et al. The impact of a single bout of high intensity circuit training on myokines’ concentrations and cognitive functions in women of different age. Physiol Behav. Elsevier Inc. 2017;179:290–7.
Gmiąt A, Jaworska J, Micielska K, Kortas J, Prusik K, Prusik K, et al. Improvement of cognitive functions in response to a regular Nordic walking training in elderly women – a change dependent on the training experience. Exp Gerontol. Elsevier Inc. 2018;104:105–12.
Kilian O, Hartmann S, Dongowski N, Karnati S, Baumgart-Vogt E, Härtel FV, et al. BDNF and its TrkB receptor in human fracture healing. Ann Anat. Urban und Fischer Verlag Jena. 2014;196:286–95.
Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, et al. Resistance exercise increases NF-κB activity in human skeletal muscle. Am J Physiol Integr Comp Physiol [Internet]. American Physiological Society Bethesda, MD; 2012 [cited 2020 Mar 5];302:R667–73. Available from: https://doi.org/10.1152/ajpregu.00336.2011
Kraemer WJ, Hatfield DL, Comstock BA, Fragala MS, Davitt PM, Cortis C, et al. Influence of HMB supplementation and resistance training on cytokine responses to resistance exercise. J Am Coll Nutr Routledge. 2014;33:247–55.
Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun. Academic Press Inc. 2014;39:80–6.
Coniglio SJ. Role of tumor-derived chemokines in osteolytic bone metastasis. Front Endocrinol (Lausanne). Frontiers Media S.A. 2018;9:313.
Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. American Physiological Society Bethesda, MD. 2013:330–58.
Acknowledgments
The authors would like to thank the Australian Institute for Musculoskeletal Science (AIMSS) and the Department of Medicine-Western Health, at the University of Melbourne, for supporting the authors of this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
BK, JF, GL, and GD have no conflicts of interest to declare.
Ethical Approval
This review article does not present any previously unpublished original research, and ethical approval is therefore not applicable.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
About this article
Cite this article
Kirk, B., Feehan, J., Lombardi, G. et al. Muscle, Bone, and Fat Crosstalk: the Biological Role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep 18, 388–400 (2020). https://doi.org/10.1007/s11914-020-00599-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00599-y