ABSTRACT
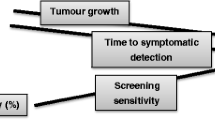
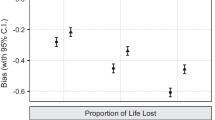
Lead-time can mean two different things: Clinical lead-time is the lead-time for clinically relevant tumors; that is, those that are not overdiagnosed. Model-based lead-time is a theoretical construct where the time when the tumor would have caused symptoms is not limited by the person’s death. It is the average time at which the diagnosis is brought forward for both clinically relevant and overdiagnosed cancers. When screening for breast cancer, clinical lead-time is about 1 year, while model-based lead-time varies from 2 to 7 years. There are two different methods to calculate overdiagnosis in cancer screening—the excess-incidence approach and the lead-time approach—that rely on two different lead-time definitions. Overdiagnosis when screening with mammography has varied from 0 to 75 %. We have explained that these differences are mainly caused by using different definitions and methods and not by variations in data. High levels of overdiagnosis of cancer have usually been explained by detection of many slow-growing tumors with long lead-times. This theory can be tested by studying if slow-growing tumors accumulate in the absence of screening, which they don’t. Thus, it is likely that the natural history of many subclinical cancers is spontaneous regression.

Similar content being viewed by others
REFERENCES
Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013;158:831–839.
Zahl P-H, Jørgensen KJ, Gøtzsche PC. Overestimated lead-time in cancer screening has led to substantial under-estimating of overdiagnosis. Br J Cancer. 2013;109:2014–2019.
Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;6, CD001877. doi:10.1002/14651858.CD001877.pub5.
Zahl P-H, Strand BH, Mæhlen J. Breast cancer incidence in Norway and Sweden during introduction of nation-wide screening: prospective cohort study. BMJ. 2004;328:921–924.
Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ. 2009;339:b2587.
Kalager M, Adami H-O, Bretthauer M, Tamimi RM. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian Screening program. Ann Intern Med. 2012;156:491–499.
Boer R, Warmerdam P, de Koning H, et al. Extra incidence caused by mammographic screening. Lancet. 1994;343:979.
Zahl P-H, Mæhlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316.
Zahl P-H, Gøtzsche P, Mæhlen J. Natural history of breast cancers detected in the Swedish mammography screening program; a cohort study. Lancet Oncol. 2011;12:1118–1124.
Zahl P-H, Mæhlen J. Overdiagnosis of breast cancer after 14 years with mammography screening. Tidsskr Nor Legeforen. 2012;132:414–417.
Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL. The Wisconsin breast cancer epidemiology simulation model. J Natl Cancer Inst Monogr. 2006;36:37–47.
Andriole GL, Crawford ED, Grubb RL III, et al. Prostate cancer screening in the randomized prostate, lung, colorectal and ovarian cancer screening trial: mortality results after 13 years follow-up. JNCI. 2012;104:125–132.
Acknowledgements
All authors declare no conflict of interest: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zahl, PH., Jørgensen, K.J. & Gøtzsche, P.C. Lead-Time Models Should Not Be Used to Estimate Overdiagnosis in Cancer Screening. J GEN INTERN MED 29, 1283–1286 (2014). https://doi.org/10.1007/s11606-014-2812-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-014-2812-2