Abstract
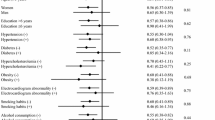
The risk factors for Parkinson’s disease (PD) are not well established. We therefore examined the prediction of various lifestyle factors on the incidence of PD in a cohort drawn from the Finnish Mobile Clinic Health Examination Survey, conducted in 1973–1976. The study population comprised 6,715 men and women aged 50–79 years and free of PD at the baseline. All of the subjects completed a baseline health examination (including height and weight measurements) and a questionnaire providing information on leisure-time physical activity, smoking, and alcohol consumption. During a 22–year follow-up, 101 incident cases of PD occurred. The statistical analyses were based on Cox’s model including age, sex, education, community density, occupation, coffee consumption, body mass index (BMI), leisure-time physical activity, smoking and alcohol consumption as independent variables. At first, BMI was not associated with PD risk, but after exclusion of the first 15 years of follow-up, an elevated risk appeared at higher BMI levels (P for trend 0.02). Furthermore, subjects with heavy leisure-time physical activity had a lower PD risk than those with no activity [relative risk (RR) 0.27, 95 % confidence interval (CI) 0.08–0.90]. In variance with findings for other chronic diseases, current smokers had a lower PD risk than those who had never smoked (RR 0.23, 95 % CI 0.08–0.67), and individuals with moderate alcohol intake (at the level of <5 g/day) had an elevated PD risk compared to non-drinkers. The results support the hypothesis that lifestyle factors predict the occurrence of Parkinson’s disease, but more research is needed.
Similar content being viewed by others
References
Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–53.
de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–35.
Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–58.
Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67(11):1955–9.
Abbott RD, Ross GW, White LR, et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology. 2002;59(7):1051–7.
Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Willett WC, Ascherio A. Obesity and the risk of Parkinson’s disease. Am J Epidemiol. 2004;159(6):547–55.
Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM. Body mass index and risk of Parkinson’s disease: a Prospective Cohort Study. Am J Epidemiol. 2007;166(10):1186–90.
Palacios N, Gao X, McCullough ML, et al. Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord. 2011;26(12):2253–9.
Ma L, Zhang L, Gao XH, et al. Dietary factors and smoking as risk factors for PD in a rural population in China: a nested case-control study. Acta Neurol Scand. 2006;113(4):278–81.
Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson’s disease. Mov Disord. 2008;23(1):69–74.
Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664–9.
Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and risk of Parkinson’s disease: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77(12):1318–22.
Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. Eur J Epidemiol. 2013;28(1):67–77.
Sasco AJ, Paffenbarger RS Jr, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson’s disease. Arch Neurol. 1992;49(4):360–5.
Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–84.
Hellenbrand W, Seidler A, Boeing H, et al. Diet and Parkinson’s disease. I: a possible role for the past intake of specific foods and food groups. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47(3):636–43.
Hernan MA, Chen H, Schwarzschild MA, Ascherio A. Alcohol consumption and the incidence of Parkinson’s disease. Ann Neurol. 2003;54(2):170–5.
Grandinetti A, Morens DM, Reed D, MacEachern D. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson’s disease. Am J Epidemiol. 1994;139(12):1129–38.
Palacios N, Gao X, O’Reilly E, et al. Alcohol and risk of Parkinson’s disease in a large, prospective cohort of men and women. Mov Disord. 2012;27(8):980–7.
Paganini-Hill A. Risk factors for Parkinson’s disease: the leisure world cohort study. Neuroepidemiology. 2001;20(2):118–24.
Willems-Giesbergen P, de Rijk M, van Swieten J, Hofman A, Breteler MM. Smoking, Alcohol, and coffee consumption and the risk of PD: results from the Rotterdam Study. Neurology. 2000;54(Suppl 3):A347–8.
Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson’s disease: a study in Swedish twins. Ann Neurol. 2005;57(1):27–33.
Sääksjärvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Männistö S. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur J Clin Nutr. 2008;62(7):908–15.
Gaig C, Tolosa E. When does Parkinson’s disease begin? Mov Disord. 2009;24(Suppl 2):S656–64.
Reunanen A, Aromaa A, Pyörälä K, Punsar S, Maatela J, Knekt P. The Social Insurance Institution’s coronary heart disease study. Baseline data and 5-year mortality experience. Acta Med Scand Suppl. 1983;673:1–120.
Malmivaara A, Heliovaara M, Knekt P, Reunanen A, Aromaa A. Risk factors for injurious falls leading to hospitalization or death in a cohort of 19,500 adults. Am J Epidemiol. 1993;138(6):384–94.
Heliövaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20(11):1830–5.
Heliövaara M. Epidemiology of sciatica and herniated lumbar intervertebral disc. ML:76. Social Insurance Institution: Helsinki; 1988. p. 49.
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76.
Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol Neurosurg Psychiatry. 2002;73(5):529–34.
Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220.
Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Weight loss in Parkinson’s disease. Ann Neurol. 2003;53(5):676–9.
Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7.
Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85(2):299–305.
Anderson RF, Harris TA. Dopamine and uric acid act as antioxidants in the repair of DNA radicals: implications in Parkinson’s disease. Free Radic Res. 2003;37(10):1131–6.
Green HJ, Fraser IG. Differential effects of exercise intensity on serum uric acid concentration. Med Sci Sports Exerc. 1988;20(1):55–9.
Poulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-parkinsonian rats. Exp Neurol. 2005;193(1):181–97.
Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–7.
Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77(3):317–21.
Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–84.
Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947–57.
Chung SJ, Armasu SM, Anderson KJ, et al. Genetic susceptibility loci, environmental exposures, and Parkinson’s disease: a case-control study of gene-environment interactions. Parkinsonism Relat Disord. 2013;19(6):595–9.
Sääksjärvi K, Knekt P, Männistö S, Heliövaara M. Self-administered questionnaire is a reliable measure of coffee consumption. J Epidemiol. 2010;20(5):363–9.
Weisskopf MG, Knekt P, O’Reilly EJ, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74(13):1055–61.
Acknowledgments
The work of K Sääksjärvi was supported by a grant from Doctoral Programs in Public Health, funded by the Ministry of Education.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sääksjärvi, K., Knekt, P., Männistö, S. et al. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol 29, 285–292 (2014). https://doi.org/10.1007/s10654-014-9887-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9887-2