Abstract
Background
The effect of Helicobacter pylori (H. pylori) eradication on gastric cancer (GC) prevention is controversial. Intestinal metaplasia (IM) seems to be a “point of no return” in the precancerous cascade. We performed a meta-analysis of randomized controlled trials (RCTs) to illustrate this issue.
Materials and Methods
The MEDLINE, EMBASE, Cochrane Library were searched for relevant RCTs that were published in any language up to March 2014. By dividing participants into subgroups based on their baseline diagnoses as group <IM (normal, non-atrophic gastritis, atrophic gastritis) and group ≥IM(intestinal metaplasia, dysplasia), the relative risk (RR) of GC in each study compared treatment group with control group were pooled using Mantel–Haenszel fixed-effect model and publication bias analyses were performed.
Results
Ten studies from eight RCTs were included in this analysis, for a total of 7,955 participants. H. pylori treatment compared with control significantly reduced the risk of GC, with a pooled RR of 0.64 (95 % CI, 0.48–0.85). Subgroup analysis for patients with non-atrophic gastritis, atrophic gastritis (<IM) yielded a similar results (RR = 0.25, 95 % CI, 0.08–0.81). But this difference was not observed in patients with intestinal metaplasia, dysplasia (≥IM) (RR = 0.88; 95 % CI, 0.59–1.31).
Conclusions
Our results suggested that patients with Intestinal metaplasia or dysplasia could not benefit from the H. pylori treatment on the risk of GC.
Similar content being viewed by others
Introduction
According to the model of gastric carcinogenesis, the consecutive precancerous lesions (PL) is usually represented as normal, non-atrophic gastritis (NAG), atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia (DYS) and gastric cancer (GC).Cure of H. pylori is proven to be effective in halting the progression of the PL and has been recommended to prevent GC [1–6]. However, the preventive effect was still controversial considering that most RCTs failed to directly demonstrate a significant decreasing on the incidence of GC after H. pylori treatment [3, 7–10]. A major consideration for the inconsistent results is a hypothetically existed time-point in the carcinogenesis process, when the histological change reached a certain degree of PL, the GC would progress anyway. Several studies have already showed that the progression of PL was more likely to be observed in individuals with a baseline diagnosis ≥IM (IM, DYS), compared with those <IM (Normal, NAG, AG). Thus, the presence of IM seems to be the “point of no return” in the process, regardless of H. pylori eradication [12–14]. However, to our knowledge, no conclusions concerning the effect of GC prevention after H. pylori eradication in these two subgroups of patients has been drawn so far.
Our study was aimed to describe the association between H. pylori eradication and GC incidence. By dividing participants from relevant RCTs into 2 subgroup based on their baseline of histological diagnoses (<IM or ≥IM), we performed a meta-analysis to compare the effect of H. pylori eradication in these two subgroups, from the perspective of preventing GC and halting PL respectively.
Methods
Search strategy
The MEDLINE, EMBASE, Cochrane Library were searched for relevant studies that were published in any language up to March 2014. The further websites were also searched, including Google Scholar, ClinicalTrials.gov, and Chinese Biomedical Literature Database (up to March 2014). We searched the literature by using the following key words and/or medical subject headings (Mesh) terms: “Helicobacter pylori”[Mesh], eradication, treatment, “Stomach Neoplasms”[Mesh], gastric cancer, gastric atrophy, intestinal metaplasia, and dysplasia. In addition, the reference lists of all included studies were reviewed carefully to identify additional eligible studies.
Study selection
Studies met the following pre-specified criteria were included as the potentially relevant studies: a design of randomized trials; contained intervention group (H. pylori treatment) and control group (placebo or not); with a duration of follow-up no less than 24 months; including participants confirmed H. pylori infection before treatment; endoscopic biopsy was performed at baseline; provided the information of GC in each group.
Two authors (Chen, Wang) independently examined all included studies in full-text, and disagreements were resolved by discussion of all authors. Duplicate publications were identified when multiple articles present common author names, locations or baseline data. Reports from the same trial were linked together and the more informative articles were selected with supplemented data from related reports. Ongoing and unpublished studies were not included in this review because of insufficient information and potential risk of bias.
Quality assessment
Two authors independently evaluated the quality of each study mainly based on the methods and results, in accordance with the assessing criteria suggested by Cochrane collaboration [15]. The quality of each study was assessed by evaluating the following item: method to generate the random sequence and conceal the treatment allocation, blinding of participants, blinding of personnel, blinding of outcome assessment (in this study, namely, the endoscopist and pathologist), whether placebo was offered, whether incomplete outcome data were described, and intention-to-treat analysis.
Data extraction
Two authors independently extracted data on the following items from the selected trials: author name; year of publication; country; study design; duration of follow-up; age range of participants; ratio of male to female; primary outcome; total number of participants enrolled; number of participants in each intervention group; number of participants with complete follow-up in each intervention group; GC in each intervention group; diagnostic criteria of H.pylori infection; histologic details (baseline of histological diagnosis, biopsy numbers, biopsysites); outcome definition (histological evaluation system); H. pylori treatment/eradication (name, dosage, and duration of treatment); the status of H. pylori infection in each intervention group.
Data synthesis and statistical analysis
The measure of effect of interest is the relative risk (RR) with 95 % confidence interval (CI).To estimate the effects of allocating the H. pylori treatment, data were generally extracted in an intention-to-treat manner, except that participants refused the allocation in each study were not counted.
The crude RRs were extracted from all studies with the raw data of GC in each intervention group and pooled in an analysis to give an estimate of the effect of H. pylori eradication in GC prevention. Subgroup analyses was performed based on study population (whether including patients with a history of GC). When anintervention group of a study contains no event, we added 0.5 to each cells of the 2 × 2 table for the study to provide a more conservative estimate of effect size [16].
Participants from studies providing individual histological data were divided into two subgroups based on their baseline diagnoses (≥IM or <IM). The cancer preventive effect of H.pylori eradication was evaluated by pooling the RR of GC development in two subgroups. Impacton halting PL was expressed as the RR of the progression of PL in treatment group compared with control group, and these RRs were pooled in two separate analyses as previously described.
The Cochran’s Q statistic and the I 2 statistics were used to assess heterogeneity among all studies. For the Q statistic, a p value of less than 0.1 was considered statistically significant. Random-effects model was used if the heterogeneity exists, otherwise the Mantel–Haenszelfixed-effect model was preferred. Publication bias was assessed by the Egger’s/Begg’s test weighted regression method; a p value of less than 0.1 was considered representative of statistically significant publication bias. All analyses were performed with Stata (version 12.0; StataCorp, College station, TX).
Results
Literature Search
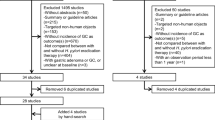
A total of 6,330 records were retrieved based on the search strategy: 3,467 from Medline, 2,737 from EMBASE, 5 from Cochrane Library, and 121 from other sources (Google scholar, ClinicalTrials.gov, Chinese Biomedical Literature Database). There were 5,016 records remained after duplicate records removed. Of these, 4,991 records were excluded based on abstracts or titles. The remaining 25 articles were reviewed in full-text. Fifteen articles were excluded for the reasons listed in Supplemental material 1, leaving a total of 10 articles from 8 trials included in this review. The study by Sung et al., which is from the same trial of Leung, was included to provide supplemental data of histological change [3, 17]. Similarly, the study by You was also included for the same reason with the article by Ma [4, 18].
Study characteristic
Characteristics of the 10 studies from 8 trials were shown in Table 1. The selected studies were published between 2000 and 2013. Four trials were conducted in China [3, 7, 9, 18], 2 in Korea [10, 19], 1 in Japan [6], and 1 in Colombia [1]. Duration of follow-up in each study ranged from 2 to 15 years. Mean age of participants ranged from 42 to 69 years. The male–female ratios were approximately 1:1 in 6 trials, 2:1 in 1 trial [10] and 3:1 in another [6]. The association between H. pylori eradication and GC was the primary outcome of interest for 5 studies, whereas it was a secondary question in the other 5 studies. The number of participants per study ranged from 169 to 2,258, for a total of 7,953 participants across all study. The proportion of participants with complete follow-up in each study ranged from 64.7 to 97.7 %. Raw data of GC in each intervention group was determined for 8 studies.
The methods to diagnose the H. pylori infection and other histologic details in each trial were shown in Table 2. Five trials chose two methods to confirm the infection while three trials used one. Participants in each study had a similar baseline of histology diagnosis, ranging from NAG to DYS, except that 3 studies included patients who had a history of GC and received endoscopic or surgical resection [6, 10, 19].
Specimens were generally obtained from antrum and corpus, except additional specimens were obtained from angulus in 3 studies [1, 7, 9]. The updated Sydney system (USS) was selected as the stipulation for the histological evaluation system of specimens in all trials except one, which used the Chinese histopathology grading criteria [4]. In most studies, GC was diagnosed once tumors invaded the lamina propria or muscularis mucosae [1, 3, 7, 9, 18]. Among studies regarded metachronous GC as outcome, two of them defined GC as either noninvasive or invasive tumor corresponding to Vienna classification [6, 10]. One used American Joint Committee on Cancer TNM classification system [19].
Five studies [1, 3, 4, 9, 18] chose the most advanced biopsy as the baseline diagnosis and two [17, 19] made the diagnosis in different sites separately, while three studies did not describe the method explicitly [19]. Regarding the definition of progression of PL, two trials [1, 4] compared the baseline and outcome histologic diagnoses based on the consecutive step of PL, two [9, 17, 19] applied a histological score comparison between baseline and outcome (e.g. progression was defined as higher score at outcome compared with baseline), while four did not provide the relevant data.
As conducted in different times, eradication therapy was not unified among the 8 serious trials (Table 3). Generally, Proton pump inhibitor triple therapy was prescribed as the initial strategy, except one, in which bismuth subsalicylate combined two kinds of antibiotics was chosen [1]. Only three trials described a design of remedies to increase the chance of successful eradication [1, 7, 18]. The mean eradication rate ranged from 46.0 to 82.5 %.
Quality assessment
The quality evaluation of included studies was shown in Table 4. Random sequence generation was explicitly specified in all trials. Allocation concealment was reported in five of the included trials. Two trials used a double-blinded design and one used a single-blinded design. Six trials explicitly specified the blinding to pathological assessment. And blinding to the endoscopist was described in four trials and was not mentioned in two trials. Half of the trials provide the placebo control to compare with H. pylori treatment. All included trials described the incomplete outcome data in each intervention group and 5 of them performed the intention-to-treat analysis. Studies included in this research present a high level of homogeneous, except three trials included patients with a history of GC. To avoid a potential risk of bias, a subgroup analysis based on these trials was performed.
Cancer preventive effect of H. pylori eradication
Among the 8 selected trials, only 2 trials suggest that H. pylori eradication can reduce the incidence of GC [6, 18]. The raw data of GC development in each intervention group were extracted from 8 studies, the crude RR ranged from 0.38 to 2.83. Overall, there were 74 cases of GC in treatment group (1.9 %, 3,992 patients) compared with 116 cases in the control group (2.9 %, 3,963 patients). In our pooled analysis, the treatment group had a reduced risk of GC development compared with control group (RR = 0.64; 95 % CI, 0.48–0.85). The impact of eradication was more pronounced on metachronous gastric cancer (RR = 0.52; 95 % CI, 0.31–0.87), compared with gastric cancer (RR = 0.70; 95 %/CI, 0.49–0.99). However, there was no significant heterogeneity between subgroups of different study population (whether including patients with a history of GC) (I 2 = 0.0 %, p = 0.609) (Fig. 1). Funnel plot asymmetry was observed (Egger’s test, p = 0.137 and Begg’s test, p = 0.035), suggesting publication bias or “small study effects”, which was most likely caused by one small study [19].
Of the eight studies comparing treatment group with control group, six reported individual baseline diagnosis in 6,873 patients [4, 7, 9–11, 19]. These patients were divided into a subgroup of ≥IM (including 4,211 patients with IM or DYS) and a subgroup <IM (including 2,662 patients with NAG or AG).In the subgroup ≥IM, 44 (2.1 %) of 2,115 patients assigned to H. pylori eradication developed GC compared with 50 (2.4 %) of 2,096 patients allocated to control group (RR = 0.88; 95 %CI, 0.59-1.31) (Fig. 2), with no significant heterogeneity between studies (I 2 = 0.0 %, p = 0.702). In the subgroup of <IM, there was 1 (0.1 %) of 1,337 patients assigned to H. pylori eradication who developed GC, compared with 11 (0.8 %) of 1,325 patients allocated to control group (RR = 0.25, 95 % CI, 0.08–0.81), with no significant heterogeneity observed (I 2 = 0.0 %, p = 0.843).Similar results were obtained in another meta-analysis with only studies of primary-prevention cohort included (Supplemental material 2).
Effect of H. pylori eradication on gastric precancerous lesions
Complete individual data of histologic change between baseline and outcome were provided by 4 studies [1, 4, 17, 19]. Patients from these four studies were divided into two groups in the same way as previously described. Eventually, there were 2,217 patients with a baseline diagnosis ≥IM (IM, DYS) and 1,623 patients <IM (NAG, AG). The RR of progression was pooled in two separate analyses with a subgroup analysis based on the definition of PL progression.
In patients with a baseline diagnosis ≥IM, results varied based on the different definition of progression, the pooled RR of progression was 1.18 (95 % CI, 1.02–1.36) in studies compared the histological step and 0.81 (95 % CI, 0.64–1.03) in studies applied a histological score comparison, with no significant heterogeneity observed (I 2 = 0.0 %, p = 0.999; I 2 = 0.0 %, p = 0.539) (Fig. 3a).
In patients with a baseline diagnosis <IM, the treatment group had a reduced risk of progression compared to the control group in studies applied a histological score comparison, with a pooled RR of 0.82 (95 % CI, 0.68–0.99). A similar but not statistically result was observed in studies compared the histological step, with a pooled RR of 0.96 (95 % CI, 0.85–1.07). There was no evidence of statistical heterogeneity of RRs across studies (I 2 = 0.0 %, p = 0.787; I 2 = 0.0 %, p = 0.930) (Fig. 3b).
Discussions
H. pylori has been given much importance in the process of gastric carcinogenesis. By including the related RCT, our meta-analysis suggests that H. pylori eradication may reduce the incidence of gastric cancer (both in primary- and secondary- prevention).In particular, for patients with a baseline diagnosis <IM, H.pylori eradication may halt the PL progression and reduce the risk of gastric cancer. However, when PL of IM or DYS present, no preventive effect was observed after eradication, neither in the risk of gastric cancer nor the PL progression.
A previous meta-analysis has already shown a reduced risk of GC after eradication. However, its result has been questioned because of redundant data [20]. We corrected it in our analysis and also included studies evaluated the effect of eradication therapy on the risk of metachronous GC (secondary preventive effect).It cannot be denied that patients with a history of GC are different from participants of other primary-prevention cohorts, with regard to genetic susceptibility, possibility of undetected malignant lesion or other unrecognized reasons. The reason why we included these secondary-prevention cohorts is that we believe the development of GC at another site of stomach could also reveal the role of H.pylori infection in the carcinogenesis. As we expected, an overall reduction of GC was observed after H. pylori eradication as well as metachronous GC, and the prophylactic power against on metachronous GC was even more obvious. Thus, we still considered the occurrence of metachronous GC as part of our analysis.
Although these conclusion have been supported by several meta-analyses [21], the controversies still exist regarding whether H. pylori eradication would be sufficient to prevent GC. For example, even with a similar research design, opposite conclusions were still drawn from these RCTs [6, 10, 19]. The baseline histological diagnosis at time of eradication is one of the major explanations for the inconsistent results, which assume that treatment before the “point of no return” in the PL may be very important. Two meta-analyses of relevant studies revealed the effect of H. pylori eradication could halt the PL progression in individuals with a baseline diagnosis <IM compared with those ≥IM, which indicated the presence of IM seems to be the “point of no return” [12, 13]. In present study, we directly compared the occurrence of GC and the results suggested that patients with IM or DYS may not benefit from the H. pylori treatment on the risk of GC. Similar results indicating that the baseline of IM was a prior risk factor of GC development after eradication can be obtained in other studies [22–24].In the aspect of primary-prevention, Mera et al. [8] prescribed eradication therapy to participants allocated in placebo group and prolong the follow up duration from 6 to 12 years, and all 9 GC patients had a baseline diagnosis of IM or DYS. A large population-based study with 4,121 participants showed the 5-year average incidence of GC decreased from 40.3 to 30.4 per 100 000 person-yearsafter eradication treatment. The incidence of AG decreased from 59.9 to 13.7 %, while the incidence of both IM and DYS increased from 40.1 to 56.1 % [14]. On the other hand, in patients without history of GC, several reports have shown the close correlation between H. pylori infection and metachronous GC occurrence [21, 25–27], however, most of them also emphasized that limited effect of eradication therapy in prevention of metachronous GC, especially in patients with IM nor DYS [28].Therefore, we believed that overall reduction of GC incidence is mainly due to the retaining of progression in patients with baseline diagnosis <IM, and malignancy transformation could hardly be prevented in those with IM or DYS.
Prospective studies, especially RCTs, which regarded the occurrence of GC as primary outcome were few, because the gastric carcinogenesis was time-consuming process which need long-term follow-up. Instead, based on the theory of consecutive progression process, many prospective studies evaluated the effect of H. pylori treatment in the PL to indirectly reflect the GC preventive effect [2, 29–36]. Though, meta-analyses [12, 13] has already concluded H. pylori treatment was succeed in halting progression of PL, we believe the retrospective design and inconsistent definitions of PL progression utilized might have a large extent influence to the pooled results. This is why most of previous RCTs assigned a single pathologist to evaluate the individual histological changes before and after eradication [1, 2, 31, 34, 37, 38].Therefore, to make a more reliable investigation, we performed an analysis only included RCTs with individual histological change provided and further conducted subgroup analyses based on the definition of PL progression (histological step or histological score).In our results, the halt effect was more obviously observed in studies applied histological score comparison with studies compared histological step. Additional, in studies used a histological step comparison, eradication seems to promote the deterioration in those with a baseline diagnosis ≥IM. As the two studies included in this analysis were both large sample randomized controlled trials, it is unlikely this result is just an incidence. We noted that both of these two studies [1, 4] have a 23 factorial design with multiple interventions given to participants, so the interaction between the H. pylori treatment and other interventions may be one explanation for this result. When we excluded other cross-over intervention groups in the study by Correa, a benefit effect was observed in the treatment group with a RR of 0.90 (95 % CI, 0.43–1.87), which may partially support our hypothesis. Anyway, regardless of the histological evaluation system used, patients with ≥IM could not benefit from H. pylori treatment.
There are three limitations of our study as shown below. Firstly, the number of studies was relatively small, and the duration of follow-up in each study varied. But all included studies were of high quality and large sample size. Second, participants included in our analysis received multiple interventions to serve as control group, such as antioxidant supplements and cyclo-oxygenase-2 inhibitor. Though our data implies the potential interaction between the H. pylori treatment and other interventions, this hypothesis is far from proved. So far, these interventions is not considered has any additional efficiency if combined with eradication treatment. Third, only the intestinal-type gastric adenocarcinoma progresses through a relatively well-defined series of histological steps. Diffuse-type gastric adenocarcinoma does not form glandular structures and is not associated with IM. Giving the low incidence of GC in these trials, further analysis focused on the histologic type of GC seems impractical. Whether different conclusion would be drawn in diffuse-type gastric adenocarcinoma need further studies.
As conclusion of the study, our results supported the effect of H. pylori eradication on both primary- and secondary- prevention. Moreover our findings suggested that patients with IM or DYS may not benefit from the H. pylori treatment on the risk of GC. Frequent endoscopic monitoring and early treatment should be considered for these patients.
Author contribution
CHN and WZ drafted the article, LX and ZZG made the study design and approved of the article. CHN and WZ contributed equally to this work
References
Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–8.
Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7–14.
Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244–9.
You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83.
Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–64.
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7.
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA J Am Med Assoc. 2004;291:187–94.
Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–40.
Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61:812–8.
Choi J, Kim SG, Yoon H, Im JP, Kim JS, Kim WH, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clinic Gastroenterol Hepatol Off Clinical Pract J Am Gastroenterol Assoc. 2014;12(793–800):e1.
Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9.
Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, et al. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253–60.
Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12(Suppl 2):32–8.
Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–82.
JPT H (eds) (2011) GS cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011). The cochrane collaboration. 2011; Available from http://www.cochrane-handbook.org. Accessed 20 May 2014
Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5.
Sung JJ, Lin SR, Leung WK. Does eradication of H. pylori prevent deterioration of gastric atrophy and intestinal metaplasia? A 5-year follow-up. Gastroenterology. 2002;122:A170.
Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–92.
Cho SJ, Choi IJ, Kook MC, Yoon H, Park S, Kim CG, et al. Randomised clinical trial: the effects of Helicobacter pylori eradication on glandular atrophy and intestinal metaplasia after subtotal gastrectomy for gastric cancer. Aliment Pharmacol Ther. 2013;38:477–89.
Ford AC, Moayyedi P. Redundant data in the meta-analysis on Helicobacter pylori eradication. Annal Int Med. 2009;151:513 (Author reply -4).
Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014;19:243–8.
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med. 2001;345:784–9.
Kim N, Park RY, Cho SI, Lim SH, Lee KH, Lee W, et al. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol. 2008;42:448–54.
Kodama M, Murakami K, Okimoto T, Abe H, Sato R, Ogawa R, et al. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand J Gastroenterol. 2013;48:1249–56.
Kim YI, Choi IJ, Kook MC, Cho SJ, Lee JY, Kim CG, et al. The association between Helicobacter pylori status and incidence of metachronous gastric cancer after endoscopic resection of early gastric cancer. Helicobacter. 2014;19:194–201.
Bae SE, Jung HY, Kang J, Park YS, Baek S, Jung JH, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. 2014;109:60–7.
Jung S, Park CH, Kim EH, Shin SJ, Chung H, Lee H, et al. Helicobacter pylori eradication is insufficient to prevent metachronous gastric lesions after ESD. J Gastroenterol. Hepatol. 2014.
Lim JH, Kim SG, Choi J, Im JP, Kim JS, Jung HC. Risk factors for synchronous or metachronous tumor development after endoscopic resection of gastric neoplasms. Gastric Cancer Off J Int Gastric Cancer Assoc Japan Gastric Cancer Assoc. 2014.
Witteman EM, Mravunac M, Becx MJ, Hopman WP, Verschoor JS, Tytgat GN, et al. Improvement of gastric inflammation and resolution of epithelial damage one year after eradication of Helicobacter pylori. J Clin Pathol. 1995;48:250–6.
Schenk BE, Kuipers EJ, Nelis GF, Bloemena E, Thijs JC, Snel P, et al. Effect of Helicobacter pylori eradication on chronic gastritis during omeprazole therapy. Gut. 2000;46:615–21.
Miwa H, Hirai S, Nagahara A, Murai T, Nishira T, Kikuchi S, et al. Cure of Helicobacter pylori infection does not improve symptoms in non-ulcer dyspepsia patients-a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2000;14:317–24.
De Leest HT, Steen KS, Bloemena E, Lems WF, Kuipers EJ, Van de Laar MA, et al. Helicobacter pylori eradication in patients on long-term treatment with NSAIDs reduces the severity of gastritis: a randomized controlled trial. J Clin Gastroenterol. 2009;43:140–6.
Kamada T, Haruma K, Hata J, Kusunoki H, Sasaki A, Ito M, et al. The long-term effect of Helicobacter pylori eradication therapy on symptoms in dyspeptic patients with fundic atrophic gastritis. Aliment Pharmacol Ther. 2003;18:245–52.
Befrits R, Sjostedt S, Tour R, Leijonmarck CE, Hedenborg L, Backman M, et al. Long-term effects of eradication of Helicobacter pylori on relapse and histology in gastric ulcer patients: a two-year follow-up study. Scand J Gastroenterol. 2004;39:1066–72.
Kuipers EJ, Nelis GF, Klinkenberg-Knol EC, Snel P, Goldfain D, Kolkman JJ, et al. Cure of Helicobacter pylori infection in patients with reflux oesophagitis treated with long term omeprazole reverses gastritis without exacerbation of reflux disease: results of a randomised controlled trial. Gut. 2004;53:12–20.
Arkkila PE, Seppala K, Farkkila MA, Veijola L, Sipponen P. Helicobacter pylori eradication in the healing of atrophic gastritis: a one-year prospective study. Scand J Gastroenterol. 2006;41:782–90.
Zullo A, Rinaldi V, Hassan C, Diana F, Winn S, Castagna G, et al. Ascorbic acid and intestinal metaplasia in the stomach: a prospective, randomized study. Aliment Pharmacol Ther. 2000;14:1303–9.
Ruiz B, Garay J, Correa P, Fontham ET, Bravo JC, Bravo LE, et al. Morphometric evaluation of gastric antral atrophy: improvement after cure of Helicobacter pylori infection. Am J Gastroenterol. 2001;96:3281–7.
Conflicts of interest
The authors declared that they have no conflicts of interest to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
H.-N. Chen and Z. Wang are contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Chen, HN., Wang, Z., Li, X. et al. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer 19, 166–175 (2016). https://doi.org/10.1007/s10120-015-0462-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0462-7