Abstract
Beta-hydroxy-beta-methylbutyrate (HMB), a metabolite of the branched-chain amino acid leucine, is extensively used by athletes and bodybuilders in order to increase strength, muscle mass and exercise performance. We performed a systematic review of the clinical literature on the effectiveness of HMB supplementation in healthy and pathological conditions (i.e. training programs, aging, acute and chronic diseases, and after bariatric surgery). We reviewed all clinical trials indexed in Medline that tested HMB supplementation as well as all the experimental data regarding HMB intracellular mechanisms of action. Search terms included: randomized controlled trials, controlled clinical trials, single- and double-blind method, HMB, proteolytic pathways, muscle atrophy, cachexia, and training. We found out 13 studies testing HMB in healthy young trained subjects, 11 in healthy young untrained subjects, 9 in patients affected by chronic diseases (i.e. cancer, HIV, chronic obstructive pulmonary disease), and 6 in elderly subjects. The indexed studies support that HMB is effective in preventing exercise-related muscle damage in healthy trained and untrained individuals as well as muscle loss during chronic diseases. Most of the selected studies showed the effectiveness of HMB in preventing exercise-related muscle damage in healthy trained and untrained individuals as well as muscle loss during chronic diseases. The usual dose of 3 g/day may be routinely recommended to maintain or improve muscle mass and function in health and disease. The safety profile of HMB is unequivocal. Further, well-designed clinical studies are needed to confirm effectiveness and mode of action of HMB, particularly in pathological conditions.
Similar content being viewed by others
Introduction
Beta-hydroxy-beta-methylbutyrate (HMB) is a five-carbon organic acid and a derivative, in vivo, of the essential amino acid leucine (LEU) via its metabolite α-ketoisocaproate (α-KIC) (Van Koevering and Nissen 1992). LEU is a potent anti-catabolic compound and a regulator of protein metabolism (Frexes-Steed et al. 1992). In fact, muscle loss in atrophic conditions can be reversed by LEU supplementation. High LEU doses counteract muscle proteolysis, while low LEU doses enhance muscle protein synthesis (Zanchi et al. 2008). Almost 80 % of LEU is normally employed for protein synthesis while the remainder is converted to α-KIC and only a small proportion of LEU (5 %) is converted into HMB (Van Koevering and Nissen 1992). As depicted in Fig. 1, LEU may be transaminated to α-KIC by two different pathways. The first one consists of transformation of α-KIC into HMB by the liver cytosolic enzyme KIC dioxygenase with cytosolic HMB subsequently converted into β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) which can be directed for cholesterol synthesis in liver and in muscle (Van Koevering and Nissen 1992). The second pathway consists of liver α-KIC oxidation into isovaleryl-CoA by the mitochondrial branched-chain ketoacid dehydrogenase (BCKD); finally, HMG-CoA is produced by the HMG-CoA synthase (Van Koevering and Nissen 1992).
HMB supplementation is claimed to exert positive effects both in healthy (i.e. increasing sport performance as well as reducing exercise-related muscle damage) and pathological conditions (i.e. preserving and increasing muscle mass) perhaps by reducing protein degradation and enhancing protein synthesis (Zanchi et al. 2010). In cachectic conditions (i.e. cancer, sepsis, acquired immunodeficiency syndrome, malnutrition, myopathies, congestive heart failure, renal failure, and chronic obstructive pulmonary disease) skeletal muscle loss is mainly consequent to a shift towards protein breakdown (Siddiqui et al. 2006). The proteolytic systems, i.e. the ubiquitin–proteasome and the caspase systems, may initiate myofibrillar proteolysis thus playing a pivotal role in muscle wasting diseases (Ventadour and Attaix 2006). HMB may influence protein metabolism as shown by changes in proteasome dependent proteolysis and protein synthesis in experimental models (Holecek et al. 2009; Kovarik et al. 2010). HMB administration to murine cell culture attenuated the tumor factor proteolysis-inducing factor (PIF)-induced activation of protein kinase C (PKC), the subsequent degradation of nuclear factor-κB inhibitor-α (IκBα) and nuclear accumulation of nuclear factor κB (NFκB). HMB also attenuated PIF-induced phosphorylation of p24/44 mitogen-activated protein kinase (MAPK) thereby interfering with proteasome expression (Smith et al. 2004). Moreover, expression of the proteasome 20S α or β subunits was reduced by 50 % as well as the ATPase subunits MSS1 and p42 of the 19S proteasome regulatory subunit and the E214k ubiquitin-conjugating enzyme (Smith et al. 2005; Nunes et al. 2008).
Muscle protein degradation also occurs following activation of caspase-3 and caspase-8 by tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), angiotensin II (ANG II) and lipopolysaccharide (LPS). The subsequent autophosphorylation and activation of protein kinase R (PKR) increases reactive oxygen species (ROS) formation via activation of p38 MAPK. ROS formation stimulates NF-κB-mediated induction of the ubiquitin–proteasome pathway. HMB completely attenuated the increase in ROS formation, caspase-3 and caspase-8 activity and PKR autophosphorylation in murine myogenic cell culture (Eley et al. 2008a; Russell and Tisdale 2009). In addition, HMB stimulated protein synthesis in murine myotubes treated with cachectic stimuli (PIF, TNF-α/IFN-γ, ANG II and LPS) through enhancement of the phosphorylation and activation of the mammalian target of rapamycin (mTOR) that, in turn, phosphorylates and activates the 70-kDa ribosomal S6 kinase (p70S6k). Cachectic stimuli also depress protein synthesis by activating PKR with subsequent phosphorylation of eukaryotic initiation factor 2 (eIF2) on the α-subunit and eukaryotic elongation factor 2 (eEF2). HMB attenuated phosphorylation of eEF2 by increasing phosphorylation of mTOR and attenuated phosphorylation of eIF2α by preventing autophosphorylation and activation of PKR. In presence of HMB, phosphorylation of the initiation factor 4E-binding protein 1 (4EBP1) was increased; so, the increased association of eukaryotic initiation factor 4E (eIF4E) with eIF4G resulted in the increase of the active eIF4G·eIF4E complex consequently to the reduction of the inactive 4E-BP1-eIF4E complex. Together, these effects active the translation machinery and attenuate PIF-induced depression of protein synthesis in murine myotubes (Eley et al. 2007, 2008b). Thus, HMB attenuation of muscle and body weight loss in experimental cancer cachexia may rely on HMB-mediated decrease in phosphorylated eIF2α and increase in phosphorylated p70S6k and phosphorylated mTOR (Aversa et al. 2011). The HMB anabolic properties are consistent with muscle hypertrophy and increase in serum insulin levels, expression of mTOR and phosphorylation of p70S6k in healthy and sedentary rats (Pimentel et al. 2011). In addition to the anabolic properties above, the increase of muscle glycogen, ATP content and citrate synthase (CS) activity after HMB supplementation to Wistar rats confirm HMB-related changes in oxidative metabolism improving muscle strength generation and performance during intense contractions (Pinheiro et al. 2012).
Tumor weight and tumor cell proliferation, ex vivo, were reduced in Walker 256 tumor-bearing rats treated with HMB (Nunes et al. 2008; Kuczera et al. 2012). These animals also expressed significant increase in IκBα, Bax/Bcl-2 protein expression ratio, phagocytic capacity and H2O2 production rates in blood polymorphonuclear cells, decrease in NFκB p65 subunit content, and an intense infiltration of leukocytes and activated granulocytes in tumor necrotic regions. HMB also decreased the extent of human peripheral blood mononuclear cell proliferation and cytokine production, in vitro (Nunes et al. 2011). However, when added to human serum-starved myoblasts HMB induced cell proliferation, MyoD (a marker for activated satellite cells) expression, the phosphorylation of mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK), muscle differentiation factors (myogenin and MEF2) expression, an increase in insulin-like growth factor-1 (IGF-1) mRNA levels and accelerated cell fusion (Kornasio et al. 2009). HMB also reduced serum-starvation-or staurosporine-induced cell apoptosis and myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats, perhaps via a reduction in pro-apoptotic protein (Bax and cleaved caspase-3) levels and increase in anti-apoptotic protein levels (Bcl-2 and Bcl-X) (Hao et al. 2011; Gerlinger-Romero et al. 2011). As the myoblast proliferation would be related to the mediation of MAPK/ERK pathway, the promotion of cell differentiation and fusion as well as the prevention of apoptosis would be related to the activation of phosphoinositide 3′-kinase (PI3 K)/Akt pathway. In fact, HMB enhances the association of the p85 subunit of PI3 K with tyrosine-phosphorylated proteins and PI3 K-dependent Akt phosphorylation thereby conditioning cell survival via inhibition of pro-apoptotic proteins (Kornasio et al. 2009). Because HMB administration increased growth hormone (GH) and IGF-I mRNA levels in vitro and in vivo models, GH/IGF-I axis may mediate some of HMB’s effects on myoblasts proliferation, differentiation and survival (Kornasio et al. 2009; Gerlinger-Romero et al. 2011).
Some of HMB’s reparative effects on damaged tissues may be due to its metabolite HMG-CoA which represents a carbon source for cholesterol synthesis allowing for cell growth, function and regenerative capability of the cell membrane (Nissen and Abumrad 1997). Therefore, HMB may prevent leakage of muscle enzymes by repairing the cell membranes damaged after physical exercise (Portal et al. 2010). In addition, HMB supplementation was recently shown to be effective in attenuating dexamethasone-induced muscle atrophy (Aversa et al. 2012) and to reduce total and low-density lipoprotein cholesterol in humans (Nissen et al. 2000). Noteworthy, despite the increase in cholesterol synthesis HMB supplementation does not influence serum testosterone levels in healthy males (Slater et al. 2000).
The objective of this paper was to systematically review the clinical effectiveness of HMB supplementation, in healthy and pathological conditions (i.e. training programs, aging, acute and chronic diseases, and after bariatric surgery). We considered available clinical trials in which HMB was administered alone or in combination with other substrates.
Methods
Search strategy
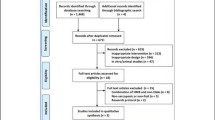
MEDLINE (www.ncbi.nlm.nih.gov/pubmed) database was searched for relevant articles by using the search terms “HMB OR beta-hydroxy-beta-methylbutyrate” AND “supplementation” AND “muscle OR muscle atrophy OR sarcopenia OR cachexia” AND “training/trained” AND “elderly” AND “proteolytic pathway(s)”. The search was restricted to human studies. The reference lists of eligible articles identified by the search were also checked to reveal other potentially relevant articles. The last literature search was completed in April 2013. A total of 39 human trials were evaluated for inclusion (Fig. 2).
Inclusion criteria
The following inclusion criteria were applied to identified clinical reports: randomized, placebo-controlled, double-blind, single-blind, not blinded trials; sample size of >5 subjects/arm; HMB supplementation, alone or in combination with other nutritional compounds, during sport, physiological conditions, cachexia, and chronic diseases; a specific dietary or nutrient intervention administered orally in the form of supplements, powder, or a beverage (non specific diets and enriched foods were excluded); published in English-language journals without considering scientific-quality index; all clinically used dosages and duration of HMB administration to humans throughout several age groups, from young subjects to old adults; the trials also had to report HMB administration conditioning on body mass (BM), fat mass (FM), fat-free mass (FFM), strength, muscle damage, inflammation, hematological/biochemical parameters, tissue regeneration. We did not include animal studies in this systematic review but we cited them in order to gather the most relevant evidences about all inquired metabolic effects, such as proteolysis, protein synthesis, cell proliferation, apoptosis, and cholesterol synthesis, in experimental, in vitro, and in vivo models. Studies that did not meet the inclusion criteria were excluded.
Studies identified by search strategy
Our search strategy identified 38 qualifying publications of which one was excluded (Nunan et al. 2010), because the supplement administered was calcium hydroxy-methylbutyrate, as also indicated by (Abumrad and Rathmacher 2011). The remaining 37 clinical trials were stratified according to the following clinical settings:
-
1.
Young adult untrained individuals;
-
2.
Young adult trained individuals;
-
3.
Elderly patients;
-
4.
Chronic diseases;
-
5.
Bariatric surgery.
Two studies (Panton et al. 2000; Nissen et al. 1996) assessed two different clinical settings, i.e. trained versus untrained subjects. Another study included healthy untrained adult males, HIV-positive subjects and cancer patients (Rathmacher et al. 2004). The effects of HMB administration alone or in combination with other nutrients, such as arginine (ARG), glutamine (GLN), lysine (LYS), creatine (CR), and α-ketoisocaproic acid (α-KIC), were analyzed separately. Meta-analysis was not feasible due to the considerable variation in study design, the type and timing of HMB intervention, the outcomes assessed, and the timing of assessments. Further details of the included studies are summarized and ordered according to the supplement type in Table 1.
Results
HMB in young trained subjects
Several studies tested the metabolic effects of HMB supplementation to young trained subjects. Augmentation of muscle mass, strength and anaerobic properties with no effects on aerobic capacity and hormonal and inflammatory mediators during the initial phases of the training season by HMB administration to elite adolescent volleyball players (Portal et al. 2011). Pre-exercise HMB supplementation significantly lowered lactate dehydrogenase (LDH) and creatine kinase (CK) activities thus mitigating exercise-induced muscle damage (Knitter et al. 2000). Maximal oxygen consumption (\( \dot V \)O2 peak) and lactate accumulation peak were unaffected by HMB administration to endurance-trained cyclists, but HMB resulted in a greater time to reach \( \dot V \)O2 peak and an increase in the onset of blood lactate accumulation (OBLA) (Vukovich and Dreifort 2001). Also HMB free acid supplementation was shown to improve markers of exercise-induced muscle damage and ameliorated recovery in resistance-trained men (Wilson et al. 2013).
On the contrary, a trivial effect on combined averaged strength measures, FM, BM and FFM was observed with HMB supplementation during resistance training (Thomson et al. 2009). Moreover, no short duration HMB-related ergogenic benefit during high-intensity training, nor significant differences between supplemental and placebo groups as for anaerobic power, CK, cortisol, testosterone and myoglobin levels were shown (Hoffman et al. 2004). Additionally, three different studies failed to demonstrate HMB-related influences on muscle function and damage parameters during a strenuous exercise program (Ransone et al. 2003; Slater et al. 2001; Kreider et al. 1999).
HMB in young untrained subjects
Three g/day of HMB supplementation to untrained subjects promoted a greater increase in FFM and peak isometric torque while a larger dose (6 g/day) produced a greater increase in peak isokinetic torque (Gallagher et al. 2000a) without compromising liver function, renal function, immune system or lipid profile during resistance training (Gallagher et al. 2000b; Wilson et al. 2009). HMB supplementation increased maximal oxygen consumption (\( \dot V \)O2max) and respiratory compensation point (RCP), i.e. components of aerobic performance (Lamboley et al. 2007), suggesting increased capacity to tolerate intense physical activity over a long period of time. Subjects receiving HMB as a free acid gel presented quicker and greater plasma concentrations and improved clearance of plasma HMB versus those receiving calcium HMB (CaHMB) gelatine capsules. This new gel formulation could improve HMB availability and efficacy to tissue (Fuller et al. 2011a).
Short-term HMB supplementation had no effect on the severity of swelling, muscle soreness or the subsequent recovery of muscle torque measures following an eccentric exercise bout (Paddon-Jones et al. 2001).
HMB in trained versus untrained subjects
A significant HMB-related increase in FFM and weight lifted with resistance training was associated with reduced 3-methylhistidine (3-MH), i.e. a marker of exercise-induced muscle proteolysis, and CK, a marker of muscle damage has been noticed (Nissen et al. 1996). Panton et al. (2000) demonstrated a greater increase in upper body strength when combined with an exercise program; plasma CK levels tended to be suppressed in the HMB group. Moreover, the HMB group tended to increase FFM and decrease percent fat. These studies found no significant influence of prior training status (trained versus untrained) or gender on the effects of HMB on body composition, strength and muscle damage (Panton et al. 2000; Nissen et al. 1996).
HMB mixed with other molecules in young trained subjects
In two different studies, no ergogenic effect of HMB or HMB/CR supplementation on muscular strength, endurance, leg power, aerobic and anaerobic ability or anthropometry were measured in rugby players over a 6-weeks resistance training program (O’Connor and Crowe 2003, 2007). There were no adverse effects on indices of health although blood bicarbonate, blood monocytes and lymphocytes that were significantly different from the control but still within normal ranges (Crowe et al. 2003).
HMB mixed with other molecules in young untrained subjects
An amino acid-based formula (containing HMB, ARG, GLN, taurine and dextrose) significantly augmented the positive benefits (improved FFM, muscle strength and muscle power) by 12 weeks of resistance exercise when compared with the placebo group. This nutritional formula promoted increased resting and exercise-induced testosterone and resting GH concentrations and reduced pre-exercise cortisol concentrations thereby improving the anabolic-to-catabolic hormone ratio. Moreover, it was associated with a decrease in plasma CK, malondialdehyde (MDA, i.e. a marker of free radical formation and lipid peroxidation which are responsible for exercise-induced membrane disruption) and percent body fat (Kraemer et al. 2009). Another study revealed a significant effect in FFM gains with CR supplementation and a trend with HBM supplementation during a weight-training program; CR- and HBM-related effects on FFM were additive. Creatine, HMB, CR/HMB supplementation caused accumulative strength increases above the placebo group. HMB alone significantly suppressed the exercise-induced rise in serum CK but CR antagonized this effect. HMB also decreased urine urea nitrogen (UUN) and blood urea nitrogen (BUN) which were not affected by CR supplementation (Jówko et al. 2001). HMB/KIC supplementation for 14 days before a single bout of eccentrically biased resistance exercise significantly attenuated the CK response, the percentage decrement in concentric one repetition maximum (1RM), the percentage increase in limb girth and delayed onset muscle soreness (DOMS) that are signs and symptoms of exercise-induced muscle damage (van Someren et al. 2005).
HMB in older adults
Quite surprisingly, studies evaluating the effects of HMB alone in old adults were limited.
HMB supplementation for 2–4 weeks was able to reduce muscle breakdown in bed-ridden old adults receiving tube feeding. Interestingly, the HMB-supplemented group showed a significant increase in waist and calf circumference and a significant decrease in UUN excretion and BUN (Hsieh et al. 2010). HMB supplementation during an exercise program tended to increase FFM gain and increased the percentage of body fat loss compared with the placebo group (Vukovich et al. 2001b).
HMB mixed with other molecules in older adults
HMB/ARG/LYS supplementation for 1 year led to a significant increase of 1.2 % in FFM, an increase of 1.6 % in body cell mass (BCM), and 8 and 12 % increases in the rates of whole body protein turnover, at 3 and 12 months, respectively (Baier et al. 2009). Improvement in the “get-up-and-go” functionality test after HMB/ARG/LYS supplementation associated with increased limb circumference, leg strength, handgrip strength, whole-body protein synthesis (plus 20 %) with positive trends in FFM was shown (Flakoll et al. 2004). The same combination increased muscle mass regardless of vitamin D status, but strength increases were observed only when subjects had adequate vitamin D status (Fuller et al. 2011b). The HMB/ARG/GLN mixture was also effective on wound collagen deposition, expressed by hydroxyproline, with no effect on total protein deposition (Williams et al. 2002).
HMB in acute and chronic diseases
A significant improvement in nitrogen balance was described in HMB-supplemented adult trauma patients but this effect was not a result of lowered muscle protein turnover as the overall Systemic Inflammatory Response Syndrome (SIRS) trended to be lower but did not reach statistical significance (Kuhls et al. 2007). A significant decrease in white blood cell count, C-reactive protein (CRP) and creatinine and a significant increase in plasma cholesterol and total protein after HMB supplementation was observed in chronic obstructive pulmonary disease (COPD) patients (Hsieh et al. 2006). BUN showed a moderate decrease after supplementation. The number of subjects with improved pulmonary function was higher in the HMB group (ten subjects) than the control group (four subjects) (Hsieh et al. 2006). Based on these observations, an anti-inflammatory and anti-catabolic effect of HMB in COPD patients in an intensive care unit setting was advocated (Hsieh et al. 2006).
HMB mixed with other molecules in chronic diseases
Interesting results were observed during cancer, notably, patients with advanced (stage IV) cancer receiving a HMB/ARG/GLN supplement gained 0.95 ± 0.66 kg of BM in 4 weeks and a change in body composition (FFM increase of 1.12 ± 0.68 kg). The FFM increase was maintained over the 24 weeks (May et al. 2002). Human immunodeficiency virus (HIV)-infected patients gained 3.0 ± 0.5 kg of BM after 8 weeks of HMB/ARG/GLN supplementation, mainly FFM (2.55 ± 0.75 kg). HMB/ARG/GLN supplementation also improved immune status, measured by increasing CD3 and CD8 cells and decreasing the HIV viral load. The HMB/ARG/GLN-supplemented group presented an increased BUN concentration due to higher nitrogen intake and trends towards lower triglyceride levels, higher protein and higher hemoglobin levels not due to treatment (Clark et al. 2000). Regarding haemoglobin, the magnitude of the response to HMB/ARG/GLN was more apparent in cancer patients compared to HIV patients and healthy volunteers. These results show that HMB/ARG/GLN can be safely used to treat AIDS- and cancer-related muscle wasting (Rathmacher et al. 2004). Berk et al. (2008) showed a strong trend towards higher FFM and BM in HMB/ARG/GLN-supplemented patients but they did not adequately test the ability of HMB/ARG/GLN to reverse or prevent cancer cachexia because most of patients did not complete the study. It was also demonstrated that both placebo and experimental amino acid mixtures significantly increased FFM, total body protein, arms and legs lean mass, and measures of physical function in rheumatoid arthritis patients but HMB/ARG/GLN supplementation was not superior to placebo in reversing rheumatoid cachexia (Marcora et al. 2005). No influence of HMB/ARG/GLN administration on the decrease in BM, body mass index (BMI), FFM and resting metabolic rate (RMR) after laparoscopic gastric bypass (LGB) was observed. Therefore, there was no potential preservation of FFM (Clements et al. 2011; Breitman et al. 2011). In addition, no effects on the early postoperative incretins after LGB, negative influence on insulin sensitivity, and degree of inflammatory markers after HMB/ARG/GLN administration were measured (Breitman et al. 2011).
Discussion
This systematic review reports the effects of HMB supplementation on body composition, in particular on muscle function, metabolic and inflammatory indices and quality of life in health and disease. The studies showing overall positive effects outnumbered those showing no response; we found positive results both in healthy subjects and in patients with different pathological conditions (Table 1).
The selected studies showed the effectiveness of HMB in preventing exercise-related muscle damage in healthy trained and untrained individuals as well as muscle loss during chronic diseases.
Dose and safety of treatment
Normally, an individual metabolizes 60 g of L-LEU to obtain 3 g of HMB but a 70 kg person produces 0.2–0.4 g of HMB per day, depending on the dose of LEU in the diet (Van Koevering and Nissen 1992). The dose of HMB provided to the treatment groups varied between trials but in the majority 3 g/day of HMB were provided (Table 1). Up to 3 g of HMB could improve strength and FFM and reduce muscle damage in a dose dependent manner, while higher doses, such as 6 g, had no additional benefits. The 3 g (or 38 mg/kg of body weight per day) dose may be an optimal dosage but too few studies have investigated the efficacy of higher dosages of HMB (Kreider et al. 1999; Gallagher et al. 2000a, b). Currently, all studies reported no adverse effects from the daily use of HMB. Renal, hematological, hepatic, endocrine functions were not negatively affected as a result of the intake of the nutrient supplement as well as any marker of tissue damage (Table 1). BUN increase was reported in only two studies (Rathmacher et al. 2004; Clark et al. 2000). This effect was possibly caused by the additional nitrogen consumed or perhaps ureagenesis induced by arginine and glutamine supplementation.
Vukovich et al. (2001a) determined the influence of oral glucose ingestion upon the time course kinetics of HMB in humans. This study reported no major differences between concomitant glucose/HMB and HMB supplementation alone, except for the longer interval required for the HMB concentration to peak and the longer plasma half-life when HMB was consumed with glucose. All studies employed capsule, drink or powder form of CaHMB salt (Table 1). Two studies used HMB free acid form, which produced a different plasma kinetic profile (Fuller et al. 2011a, b; Wilson et al. 2013). CaHMB has a potential role as a phosphate binder in uremia as demonstrated in vitro. It might contribute to the management of hyperphosphatemia in uremic patients (Sousa et al. 1996). In one recent experimental study it was observed that HMB supplementation induced hyper-insulinemia (Gerlinger-Romero et al. 2011).
Timing and duration of the intervention supplementation
The optimal timing of the HMB supplementation during exercise has not been established. Several studies employed HMB during training and a small number of studies on pre- and post- physical exercise, in chronic diseases, and in old adults.
HMB was administered when the wasting condition was already present. Therefore, additional studies to establish if HMB is recommended to prevent protein-energy wasting are strongly needed.
Study quality and choice of control groups
Variations in study design contributed to the lack of consistency in results. The quality of gathered clinical trials is justified by their MEDLINE publication, adequate blinding of allocation, and adequate blinding of investigators to treatment group in almost all studies. The differences in outcomes as well as sociodemographic characteristics between participants who were followed-up or lost to follow-up because of dropping out are well documented. Sometimes this selective loss limited statistical power as well as the presence of different bias. Differences in the outcomes assessed and the time of assessment likely contributed to variability in the results. In most studies, HMB was not used alone but in combination with ARG, GLN, LYS, CR, α-KIC, ascorbic acid, taurine, sugar and dextrose. These molecules may have increased or conditioned HMB effects on body metabolism thereby making difficult to determine the individual role of HMB and other nutritional principles.
Conclusions
The effects of HMB on cell metabolism and dynamics influenced several metabolic changes, such as enhancement of whole body protein synthesis, increased collagen synthesis, inhibition of protein degradation and increase in muscle cell membrane cholesterol synthesis.
HMB supplementation contributed to preserve FFM in cancer, AIDS, elderly, and following trauma. It also improved contractile performance as well as endurance aerobic performance and muscle strength. It also reduced exercise-induced markers of muscle damage, post-exercise recovery time and improved quality of life and respiratory function in COPD.
Further studies are necessary to indicate the optimal dosage in consideration of the different damage and catabolic stimuli, the optimal duration of supplementation and the best form of supplementation, i.e. gel, powder, capsule or drink, taking into account the HMB intestinal rates of HMB absorption, metabolism, plasma kinetics and interactions with other supplements. The effects obtained with short-lasting HMB supplementation appear trivial in comparison with long-lasting HMB supplementation; a long-lasting HMB supplementation is able to counteract muscle loss in cachectic conditions as well as to prevent muscle damage and support muscular strength in trained and untrained individuals engaging in resistance-exercise training (Wilson et al. 2008). Future trials should also investigate the cost/effectiveness of HMB supplementation (alone or in combination with other substrates) in the prevention/treatment of pathologic loss of muscle mass and function in different clinical settings.
The selected studies showed the effectiveness of HMB in preventing exercise-related muscle damage in healthy trained and untrained individuals as well as muscle loss during chronic diseases. The usual dose of 3 g/day may be routinely recommended to maintain or improve muscle mass and function in health and disease. The safety profile of HMB is unequivocal. Further, well designed clinical studies are needed to confirm effectiveness and mode of action of HMB.
References
Abumrad NN, Rathmacher JA (2011) Exercise-induced muscle damage is not attenuated by maximuscle β-hydroxy-β-methylbutyrate-1000™ supplementation. J Stren Cond Res 25:1–2
Aversa Z, Bonetto A, Costelli P et al (2011) Beta-hydroxy-beta-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Onc 38:713–720
Aversa Z, Alamdari N, Castillero E et al (2012) β-hydroxy-β-methylbutyrate (hmb) prevents dexamethasone-induced myotube atrophy. Biochem Biophys Res Commun 423:739–743
Baier S, Johannsen D, Abumrad N et al (2009) Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), l-arginine, and l-lysine. JPEN J Parenter Enter Nutr 33:71–82
Berk L, James J, Schwartz A et al (2008) RTOG. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Supp Care Can 16:1179–1188
Breitman I, Saraf N, Kakade M et al (2011) The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass. J Am Coll Surg 212:617–625
Clark RH, Feleke G, Din M et al (2000) Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enter Nutr 24:133–139
Clements RH, Saraf N, Kakade M et al (2011) Nutritional effect of oral supplement enriched in beta-hydroxy-beta-methylbutyrate, glutamine and arginine on resting metabolic rate after laparoscopic gastric bypass. Surg Endosc 25:1376–1382
Crowe MJ, O’Connor DM, Lukins JE (2003) The effects of beta-hydroxy-beta-methylbutyrate (HMB) and HMB/creatine supplementation on indices of health in highly trained athletes. Int J Sport Nutr Exer Metab 13:184–197
Eley HL, Russell ST, Baxter JH et al (2007) Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab 293:E923–E931
Eley HL, Russell ST, Tisdale MJ (2008a) Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab 295:E1409–E1416
Eley HL, Russell ST, Tisdale MJ (2008b) Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab 295:E1417–E1426
Flakoll P, Sharp R, Baier S et al (2004) Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 20:445–451
Frexes-Steed M, Lacy DB, Collins J et al (1992) Role of leucine and other amino acids in regulating protein metabolism in vivo. Am J Physiol 262:E925–E935
Fuller JC Jr, Sharp RL, Angus HF et al (2011a) Free acid gel form of β-hydroxy-β-methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br J Nutr 105:367–372
Fuller JC, Baier S, Flakoll P et al (2011b) Vitamin D status affects strength gains in older adults supplemented with a combination of β-hydroxy-β-methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enter Nutr 35:757–762
Gallagher PM, Carrithers JA, Godard MP et al (2000a) Beta-hydroxy-beta-methylbutyrate ingestion, part I: effects on strength and fat free mass. Med Sci Sports Exerc 32:2109–2115
Gallagher PM, Carrithers JA, Godard MP et al (2000b) Beta-hydroxy-beta-methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Med Sci Sports Exerc 32:2116–2119
Gerlinger-Romero F, Guimarães-Ferreira L, Giannocco G et al (2011) Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Grow Horm IGF Res 21:57–62
Hao Y, Jackson JR, Wang Y et al (2011) β-hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Reg Integ Comp Physiol 301:R701–R715
Hoffman JR, Cooper J, Wendell M et al (2004) Effects of beta-hydroxy beta-methylbutyrate on power performance and indices of muscle damage and stress during high-intensity training. J Stren Cond Res 18:747–752
Holecek M, Muthny T, Kovarik M et al (2009) Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol 47:255–259
Hsieh LC, Chien SL, Huang MS et al (2006) Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr 15:544–550
Hsieh LC, Chow CJ, Chang WC et al (2010) Effect of beta-hydroxy-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac J Clin Nutr 19:200–208
Jówko E, Ostaszewski P, Jank M et al (2001) Creatine and beta-hydroxy-beta-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program. Nutrition 17:558–566
Knitter AE, Panton L, Rathmacher JA et al (2000) Effects of beta-hydroxy-beta-methylbutyrate on muscle damage after a prolonged run. J Appl Physiol 89:1340–1344
Kornasio R, Riederer I, Butler-Browne G et al (2009) Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3 K/Akt pathways. Biochim Biophys Acta 1793:755–763
Kovarik M, Muthny T, Sispera L et al (2010) Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J Physiol Biochem 66:311–319
Kraemer WJ, Hatfield DL, Volek JS et al (2009) Effects of amino acids supplement on physiological adaptations to resistance training. Med Sci Sports Exerc 41:1111–1121
Kreider RB, Ferreira M, Wilson M et al (1999) Effects of calcium beta-hydroxy-beta-methylbutyrate (HMB) supplementation during resistance-training on markers of catabolism, body composition and strength. Int J Sports Med 20:503–509
Kuczera D, Paro de Oliveira HH, Fonseca Guimarães Fde S et al (2012) Bax/Bcl-2 protein expression ratio and leukocyte function are related to reduction of walker-256 tumor growth after β-hydroxy-β-methylbutyrate (HMB) administration in wistar rats. Nutr Can 64:286–293
Kuhls DA, Rathmacher JA, Musngi MD et al (2007) Beta-hydroxy-beta-methylbutyrate supplementation in critically ill trauma patients. J Trauma 62:125–132
Lamboley CR, Royer D, Dionne IJ (2007) Effects of beta-hydroxy-beta-methylbutyrate on aerobic-performance components and body composition in college students. Int J Sport Nut Exer Metab 17:56–69
Marcora S, Lemmey A, Maddison P (2005) Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr 24:442–454
May PE, Barber A, D’Olimpio JT et al (2002) Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 183:471–479
Nissen SL, Abumrad NN (1997) Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J Nutr Biochem 8:300–311
Nissen S, Sharp R, Ray M et al (1996) Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol 81:2095–2104
Nissen S, Sharp RL, Panton L et al (2000) Beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 130:1937–1945
Nunan D, Howatson G, van Someren KA (2010) Exercise-induced muscle damage is not attenuated by beta-hydroxy-beta-methylbutyrate and alpha-ketoisocaproic acid supplementation. J Stren Cond Res 24:531–537
Nunes EA, Kuczera D, Brito GA et al (2008) Beta-hydroxy-beta-methylbutyrate supplementation reduces tumor growth and tumor cell proliferation ex vivo and prevents cachexia in walker 256 tumor-bearing rats by modifying nuclear factor-kappaB expression. Nutr Res 28:487–493
Nunes EA, Lomax AR, Noakes PS et al (2011) β-hydroxy-β-methylbutyrate modifies human peripheral blood mononuclear cell proliferation and cytokine production in vitro. Nutrition 27:92–99
O’Connor DM, Crowe MJ (2003) Effects of beta-hydroxy-beta-methylbutyrate and creatine monohydrate supplementation on the aerobic and anaerobic capacity of highly trained athletes. J Sports Med Phys Fit 43:64–68
O’Connor DM, Crowe MJ (2007) Effects of 6 weeks of beta-hydroxy-beta-methylbutyrate (HMB) and HMB/creatine supplementation on strength, power, and anthropometry of highly trained athletes. J Stren Cond Res 21:419–423
Paddon-Jones D, Keech A, Jenkins D (2001) Short-term beta-hydroxy-beta-methylbutyrate supplementation does not reduce symptoms of eccentric muscle damage. Int J Sport Nut Exer Metab 11:442–450
Panton LB, Rathmacher JA, Baier S et al (2000) Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (HMB) during resistance training. Nutrition 16:734–739
Pimentel GD, Rosa JC, Lira FS et al (2011) β-hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab 8:11
Pinheiro CH, Gerlinger-Romero F, Guimarães-Ferreira L et al (2012) Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur J Appl Physiol 112:2531–2537
Portal S, Eliakim A, Nemet D et al (2010) Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices. J Pediatr Endocrinol Metab 23:641–650
Portal S, Zadik Z, Rabinowitz J et al (2011) The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double-blind, placebo-controlled study. Eur J Appl Physiol 111:2261–2269
Ransone J, Neighbors K, Lefavi R et al (2003) The effect of beta-hydroxy beta-methylbutyrate on muscular strength and body composition in collegiate football players. J Stren Cond Res 17:34–39
Rathmacher JA, Nissen S, Panton L et al (2004) Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enter Nutr 28:65–75
Russell ST, Tisdale MJ (2009) Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem 330:171–179
Siddiqui R, Pandya D, Harvey K et al (2006) Nutrition modulation of cachexia/proteolysis. Nut Clin Prac 21:155–167
Slater GJ, Logan PA, Boston T et al (2000) Beta-hydroxy beta-methylbutyrate (HMB) supplementation does not influence the urinary testosterone: epitestosterone ratio in healthy males. J Sci Med Sport 3:79–83
Slater G, Jenkins D, Logan P et al (2001) Beta-hydroxy-beta-methylbutyrate (HMB) supplementation does not affect changes in strength or body composition during resistance training in trained men. Int J Sport Nut Exer Metab 11:384–396
Smith HJ, Wyke SM, Tisdale MJ (2004) Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Can Res 64:8731–8735
Smith HJ, Mukerji P, Tisdale MJ (2005) Attenuation of proteasome-induced proteolysis in skeletal muscle by (beta)-hydroxy-(beta)-methylbutyrate in cancer-induced muscle loss. Can Res 65:277–283
Sousa MF, Abumrad NN, Martins C et al (1996) Calcium beta-hydroxy-beta-methylbutyrate 1. Potential role as a phosphate binder in uremia: in vitro study. Nephron 72:391–394
Thomson JS, Watson PE, Rowlands DS (2009) Effects of 9 weeks of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in resistance trained men. J Stren Cond Res 23:827–835
Van Koevering M, Nissen S (1992) Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta methylbutyrate in vivo. Am J Physiol 262:E27–E31
van Someren KA, Edwards AJ, Howatson G (2005) Supplementation with beta-hydroxy-beta-methylbutyrate (HMB) and alpha-ketoisocaproic acid (KIC) reduces signs and symptoms of exercise-induced muscle damage in man. Int J Sport Nut Exer Metab 15:413–424
Ventadour S, Attaix D (2006) Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 18:631–635
Vukovich MD, Dreifort GD (2001) Effect of beta-hydroxy beta-methylbutyrate on the onset of blood lactate accumulation and V(O)(2) peak in endurance-trained cyclists. J Stren Cond Res 15:491–497
Vukovich MD, Slater G, Macchi MB et al (2001a) Beta-hydroxy-beta-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J Nutr Biochem 12:631–639
Vukovich MD, Stubbs NB, Bohlken RM (2001b) Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J Nutr 131:2049–2052
Williams JZ, Abumrad N, Barbul A (2002) Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg 236:369–375
Wilson GJ, Wilson JM, Manninen AH (2008) Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nut Metab 5:1–17
Wilson JM, Kim JS, Lee SR et al (2009) Acute and timing effects of beta-hydroxy-beta-methylbutyrate (HMB) on indirect markers of skeletal muscle damage. Nut Metab 6:1–8
Wilson JM, Lowery RP, Joy JM et al (2013) β-hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br J Nutr 3:1–7
Zanchi NE, Nicastro H, Lancha AH Jr (2008) Potential antiproteolytic effects of l-leucine: observations of in vitro and in vivo studies. Nut Metab 5:20
Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L et al (2010) HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids 40:1015–1025
Acknowledgments
We are grateful to Joseph Lee Beverly, PhD, Professor and Chair, Department of Nutrition, University of North Carolina at Greensboro, USA for his invaluable help in the critical revision of this manuscript and to Lorenzo Maria Donini, MD, Professor of Nutrition, Sapienza University of Rome, for his helpful methodological advice. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Molfino, A., Gioia, G., Rossi Fanelli, F. et al. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids 45, 1273–1292 (2013). https://doi.org/10.1007/s00726-013-1592-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1592-z