Abstract
Purpose
Oral mucositis induced by radiation or chemoradiation can cause devastating quality of life issues for patients undergoing treatment for head and neck cancer. In this study, we investigated the efficacy of a traditional Japanese medicine—Hangeshashinto (TJ-14)—for (chemo)radiation-induced oral mucositis.
Methods
Eighty patients who underwent whole neck radiation of >60 Gy with or without chemotherapy (high-dose cisplatin or low-dose docetaxel) were enrolled in this retrospective study; 40 had received TJ-14 during treatment, and 40 had not (controls). Factors related to alleviation of oral mucositis were identified by multivariate logistic regression analysis. Rates of completion of (chemo)radiation treatments were compared between the patients who received TJ-14 and the control group according to the treatment regimen. The comparison of the nutrition status between groups was also performed.
Results
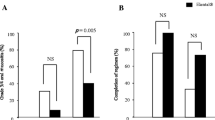
Multivariate analysis indicated that the use of TJ-14 (p = 0.019), gender (p = 0.024), and primary tumor location (p = 0.028) were significant factors associated with the severity of oral mucositis. TJ-14 was associated with a significantly improved rate of completion of chemoradiation with cisplatin (p = 0.002). In the investigation of nutritional status, only serum albumin was significantly maintained better in the TJ-14 group than the control group in terms of mean change before and after (chemo)radiation (p = 0.024).
Conclusions
The present study indicates that TJ-14 is effective for ameliorating oral mucositis induced by (chemo)radiation in patients with head and neck cancers. TJ-14 was associated with improved completion rates of chemoradiation treatments with cisplatin. A randomized controlled trial is necessary to confirm the efficacy of TJ-14 for chemoradiation-induced mucositis in head and neck cancer patients.

Similar content being viewed by others
References
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Bourhis J, Lapeyre M, Tortochaux et al (2011) Accelerated radiotherapy and concomitant high dose chemotherapy in non resectable stage IV locally advanced HNSCC: results of a GORTEC randomized trial. Radiother Oncol 100:56–61. doi:10.1016/j.radonc.2011.07.006
Sonis ST (2011) Oral mucositis. Anti-Cancer Drugs 22:607–612
Robertson C, Robertson AG, Hendry JH et al (1998) Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int J Radiat Oncol Biol Phys 40:319–329
Bese NS, Hendry J, Jeremic B (2007) Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 68:654–661
Withers HR, Taylor JM, Maciejewski B (1988) The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27:131–146
Herrmann T, Jakubek A, Trott KR (1994) The importance of the timing of a gap in radiotherapy of squamous cell carcinomas of the head and neck. Strahlenther Onkol 170:545–549
Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B (2003) Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys 56:399–412
Russo G, Haddad R, Posner M, Machtay M (2008) Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 13:886–898. doi:10.1634/theoncologist 2008–0024
Sonis ST, Oster G, Fuchs H et al (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19:2201–2205
Keefe DM, Schubert MM, Elting LS et al (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Peterson DE, Bensadoun RJ, Roila F et al (2009) Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol 20:174–177
Quinn B, Potting CM, Stone R et al (2008) Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplantation patients. Eur J Cancer 44:61–72
Henke M, Alfonsi M, Foa P et al (2011) Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol 29:2815–2820. doi:10.1200/JCO.2010.32.4103. Epub 2011 Jun 13
Bensinger W, Schubert M, Ang KK et al (2008) NCCN Task Force Report. Prevention and management of mucositis in cancer care. J Natl Compr Canc Netw 6(1):S1–21, quiz S22-4
Svanberg A, Ohrn K, Birgegard G (2010) Oral cryotherapy reduces mucositis and improves nutrition—a randomised controlled trial. J Clin Nurs 19:2146–2151. doi:10.1111/j.1365-2702.2010.03255.x
Scully C, Epstein J, Sonis S et al (2004) Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck 26:77–84
Cowen D, Tardieu C, Schubert M et al (1997) Low energy Helium-Neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. Int J Radiat Oncol Biol Phys 38:697–703
Cheah KY, Howarth GS, Yazbeck R et al (2009) Grape seed extract protects IEC-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol Ther 8:382–390
Cheah KY, Bastian SE, Acott TM et al (2013) Grape seed extract reduces the sevility of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Dig Dis Sci 58:970–977
Wright TH, Yazbeck R, Lymn KA et al (2009) The herbal extract, Iberogast®, improves jejunal integrity in rats with 5-fluorouracil (5-FU)-induced mucositis. Cancer Biol Ther 8:923–929
Kono T, Satomi M, Chisato N et al (2010) Topical application of Hangeshashinto (TJ-14) in the treatment of chemotherapy- induced oral mucositis. World J Oncol 1:232–235
Gibson RJ, Keefe DMK, Lalla RV et al (2013) Systemic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 21:313–326
Bensinger WB, Schubert M, Ang KK et al (2008) NCCN task force report: prevention and management of mucositis in cancer care. J Natl Compr Canc Netw 6(1):S1–S23
Hensley ML, Hagerty KL, Kewalramani T et al (2009) American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 27:127–145
Peterson DE, Bensadoun RJ, Rolia F (2011) Management of oral and gastrointestinal mucositis: ESMO clinical practice guidelines. Ann Oncol 22(6):vi78–vi84
Le QT, Kim HE, Schneider CJ et al (2011) Palifermin reduces severe mucositis in definitive chemoradiation of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol 29:2808–2814
Bossi P, Locati LD, Licitra L (2012) Palifermin in prevention of head and neck cancer radiation-induced mucositis: not yet a definitive word on safety and efficacy profile. J Clin Oncol 30(564–565):565–567. doi:10.1200/JCO.2011.39.1136
Satoh H, Ishikawa H, Murakami O et al (1999) Reduction by Hange-Shashin-To (TJ-14), a herbal medicine on gastrointestinal tract complications induced by CPT-11 containing chemotherapy. Cancer Res Ther Contr 7:321–323
Sonis ST (2004) A biological approach to mucositis. J Support Oncol 2:21–36
Samosorn S, Tanwirat B, Muhamad N et al (2009) Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg Med Chem 17:3866–3872
Grycova L, Dostal J, Marek R et al (2007) Quaternary protoberberine alkaloids. Phytochemistry 68:150–175
Hattori T, Nagamatsu T, Ito M, Suzuki Y (1989) Studies on antinephritic effect of TJ-8014, a new Japanese herbal medicine, and its mechanisms (2): effect on the release of corticosterone from adrenal glands. Jpn J Pharmacol 51:117–124
Kase Y, Saitoh K, Ishige A et al (1998) Mechanisms by which Hange-shashin-to reduces prostaglandin E2 levels. Biol Pharm Bull 21:1277–1281
Tawata M, Aida K, Noguchi T (1992) Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur J Pharmacol 212:87–92
Morimoto S, Tateishi N, Matsuda T et al (1998) Novel hydrogen peroxide metabolism in suspension cells of Scutellaria baicalensis Georgi. J Biol Chem 273:12606–12611
Kase Y, Hayakawa T, Ishige A et al (1997) The effects of Hange-shashin-to on the content of prostaglandin E2 and water absorption in the large intestine of rats. Biol Pharm Bull 20:954–957
Conflict of interest
There is no financial support for the present study, and the authors have declared no conflicts of interest. The authors are fully responsible for the content of this paper. All authors reviewed and approved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, T., Araki, K., Tomifuji, M. et al. A traditional Japanese medicine—Hangeshashinto (TJ-14)—alleviates chemoradiation-induced mucositis and improves rates of treatment completion. Support Care Cancer 23, 29–35 (2015). https://doi.org/10.1007/s00520-014-2315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2315-z