Abstract
Purpose
This study aims to explore the experiences of patients enrolled in a cancer-related clinical drug treatment trial utilising a qualitative focus-group methodology. Specifically, this study aimed to explore the impact of social and family support, the challenges and advantages of taking part in a clinical trial and the experiences of patients at the conclusion of the trial.
Methods
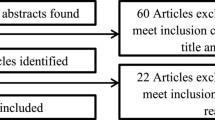
A qualitative study was conducted at a public hospital in Melbourne in 2008. A total of 14 participants were recruited. Three focus groups and two interviews were conducted with 13 patients who had completed a cancer-related clinical trial. Comments from a letter written by a trial participant were also analysed. Interviews were audio-recorded, transcribed and coded according to emerging themes.
Results
Information obtained was grouped around four main themes; making sense of trial participation, challenges of treatment in the context of clinical trial participation, support during trial participation and coping with trial conclusion. Participants experienced a mixture of hope, uncertainty and apprehension as they considered whether to take part in a clinical trial. At different stages of the trial they made sense of their participation by thinking about the possible benefits of participation. Trial participation was also associated with a number of emotional and practical challenges. Generally, participants were very positive about the support they received from health professionals, family and friends. The end of the trial was associated with a mix of emotions, including relief, disappointment, hope of future help, uncertainty and abandonment.
Conclusions
Clinical trial participation is a positive experience for many patients with cancer, although there are a number of associated practical and emotional challenges. Trial participants may benefit from closer follow-up from clinical trial staff, especially the treating doctor, assessment of support needs and help in re-evaluating the meaning of their trial participation if their initial hopes and expectations are not met.
Similar content being viewed by others
References
Cox K, Wilson E, Arthur A et al (2005) Br J Cancer 93:41–45
Cancer Australia. Support for clinical trials. Dickson, ACT: Cancer Australia. http://www.canceraustralia.gov.au/research-and-clinical-trials/support-for-clinical-trials.aspx (accessed Oct 2008).
Moore S (2001) A need to try everything: patient participation in phase I trials. J Adv Nurs 33(6):738–747
Madsen SM, Mirza MR, Holm S et al (2002) Attitudes towards clinical research amongst participants and nonparticipants. J Intern Med 25(2):156–168
Nurgat ZA, Craig W, Campbell NC et al (2005) Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer 92(6):1001–1005
Carlson LE, Bultz BD, Morris DG (2005) Individualized quality of life: standardized quality of life, and distress in patients undergoing a phase I trial of the novel therapeutic Rsolysin (reovirus). Health and Quality of Life Outcomes 3(1):7
Garcea G, Lloyd T, Steward WP et al (2005) Differences in attitudes between patients with primary colorectal cancer and patients with secondary colorectal cancer: is it reflected in their willingness to participate in drug trials? Eur J Cancer Care 14:166–170
Cox K, Avis M (1996) Psychosocial aspects of participation in early anticancer drug trials: report of a pilot study. Cancer Nurs 19(3):177–186
Cox K (1999) Researching research: patients’ experiences of participation in phase I and II anti-cancer drug trials. Eur J Oncol Nurs 3(3):143–152
Cohen MZ, Slomka J, Pentz RD et al (2007) Phase I participants’ view of quality of life and trial participation burdens. Support Care Cancer 15:885–890
Burnet K, Benson J, Earl H et al (2004) A survey of breast cancer patients’ views on entry into several cinical studies. Eur J Cancer Care 13(1):32–35
Borrel-Carrio F, Suchman AL, Epstein RM (2004) The biopsychosocial model 25 years later: principles, practice and scientific inquiry. Annals of family medicine 2:576–582
Caltabiano ML, Byrne D, Martin PR, Sarafino EP (2002) Health psychology: biopsychosocial interactions, an Australian perspective. Wiley, Milton
Murphy B, Cockburn J, Murphy M (1992) Focus groups in health research. Health Promotion Journal of Australia 2(2):37–40
Kitzinger J (1995) Qualitative research: introducing focus groups. Br Med J 311(7000):299–302
Acknowledgements
This study was funded by the Victorian Cancer Agency. Thank you to Michael Murphy from Market Access, Research and Consulting for facilitating and transcribing the focus group discussions and providing advice on qualitative data analyses. Thank you also to Pat Bugeja, Helen Crowe and Marian Lieshcke for assistance in recruiting patients into the study. We are grateful to all patients who took part in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wootten, A.C., Abbott, J.M., Siddons, H.M. et al. A qualitative assessment of the experience of participating in a cancer-related clinical trial. Support Care Cancer 19, 49–55 (2011). https://doi.org/10.1007/s00520-009-0787-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0787-z