Abstract
Objectives
Chemotherapy-induced nausea and vomiting (CINV) remains a major adverse effect of cancer chemotherapy which may increase morbidity, reduce quality of life and threaten the success of cancer therapy. Aprepitant is effective in preventing CINV, achieving higher complete response (no emesis and no rescue therapy) compared to standard prevention, in patients receiving either highly (HEC) or moderately emetogenic chemotherapy (MEC; absolute reduction = 11 and 13%, respectively). We assessed the cost effectiveness of aprepitant-based vs standard prevention in these indications in Belgium.
Materials and methods
A decision analytical model was developed in MS Excel (Fig. 1). To estimate resource use, two approaches were used. The first is based on the preventive regimens applied in randomized controlled trials comparing aprepitant-based CINV prevention (for HEC: aprepitant days 1–3, ondansetron 32 mg i.v. day 1, oral placebo twice daily days 2–4, oral dexamethasone days 1–4; for MEC: aprepitant days 1–3, ondansetron 16 mg p.o. day 1, placebo on days 2–3, oral dexamethasone day 1), vs a standard regimen (for HEC: oral placebo days 1–3, ondansetron 32 mg i.v. day 1 and 16 mg p.o. days 2–4, oral dexamethasone days 1–4; for MEC: oral placebo, ondansetron 16 mg p.o. days 1–3, dexamethasone day 1) The second analysis is based on current real-world resource use in the Belgian setting in the prevention of CINV using a longitudinal Hospital Database. CINV-specific utility values were used to calculate quality-adjusted life years (QALYs). Drug costs were obtained from official reimbursement listings. Treatment costs for CINV were obtained from a German study and adapted to Belgium.
Results
The aprepitant-based regimen is associated with 0.003 and 0.014 more QALYs in HEC and MEC, respectively and with per patient savings of €66.84 (trial based) and €74.62 (real-life based) for HEC and €17.95 (trial based) and €21.70 (real-life based) for MEC. Hence, aprepitant is both more effective and less expensive (=dominant). One-way sensitivity analyses were performed on treatment cost of emesis, the clinical benefit of aprepitant and the cost of ondansetron and showed that the results were robust on the first two parameters but sensitive on the decrease in cost of ondansetron for the moderately emetogenic chemotherapy regimens.
Conclusions
In both approaches, the aprepitant-based strategy is more effective and less expensive compared to standard care.

Similar content being viewed by others
References
Coates A, Abraham S, Kaye SB et al (1983) On the receiving end: patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19:203–208
de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, Verweij J (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76(8):1055–1061
Aapro M. S., H. J. Schmoll, S. Poli-Bigelli, K. Jordan, J. von Pawel, H. Giezek, T. Ahmed, C. Y. Chan (2005) Comparison of aprepitant combination regimen with 4-day ondansetron +4-day dexamethasone for prevention of acute and delayed nausea/vomiting after cisplatin chemotherapy. Abstract No: 8007, ASCO Annual Meeting
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersall MH (1996) On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7(2):189–195 Feb
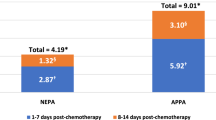
Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thodtmann J, Lordick F (2004) The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol 15(3):526–536 Mar
Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, von Pawel J, Giezek H, Ahmed T, Chan CY (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17(6):1000–1006 Jun
Herrstedt J, Muss HB, Warr DG, Hesketh PJ, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Hustad CM, Horgan KJ, Skobieranda F (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. (Trial 071 multiple cycles). Cancer 104(7):1548–1555 Oct 1
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin. J Clin Oncol 21(22):4112–4119 Nov 15
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97(12):3090–3098 Jun 15
de Wit R, Herrstedt J, Rapoport B, Carides AD, Guoguang-Ma J, Elmer M, Schmidt C, Evans JK, Horgan KJ (2004) The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomised, placebo-controlled phase III clinical trials. Eur J Cancer 40(3):403–410 Feb
Lordick F, Ehlken B, Ihbe-Heffinger A, Berger K, Krobot KJ, Pellissier J, Davies G, Deuson R (2007) Health outcomes and cost-effectiveness of aprepitant in outpatients receiving antiemetic prophylaxis for highly emetogenic chemotherapy in Germany. Eur J Cancer 43(2):299–307 Epub 2006 Nov 28, Jan
Sun C, Bodurka D, Donato M, Rubenstein E, Borden C, Basen-Engquist K, Munsell M, Kavanagh J, Gershenson D (2002) Patient preferences regarding side effects of chemotherapy for ovarian cancer: Do they change over time? Gynecol Oncol 87(1):118–120
Borjeson S, Hursti TJ, Peterson C, Fredikson M, Furst CJ, Avall-Lundqvist E, Steineck G (1997) Similarities and differences in assessing nausea on a verbal category scale and a visual analogue scale. Cancer Nurs 20(4):260–266 Aug
The Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference
Seynaeve C, Schuller J, Buser K, Porteder H, Van Belle S, Sevelda P, Christmann D, Schmidt M, Kitchener H, Pacs D, de Mulder PHM (1992) Comparison of the antiemetic efficacyof ondansetron given as either a continuousinfusion or a single intravenousdose, in acute cisplatin-induced emesis.A multicenter, double-blind, randomized,parallel group study. Br J Cancer 66:192–197
Italian Group for Antiemetic Research (IGAR) (1995) Ondansetron versus granisetron, both combined with dexamethasone,in the prevention of cisplatin-induced emesis. Ann Oncol 6:805–810
Ruff P, Paska W, Goedhals L, Pouiliart P, Riviere A, on behalf of the GranisetronStudy GroupVorolieof D, Bloch B, Jones A, Martin L, Brunet R, Butcher, Forster J, McQuade B, on behalf of theOndansetron and Granisetron EmesisStudy Group (1994) Ondansetron compared with granisetron in the prophylaxisof cisplatin-induced acute emesis: a multicenter double-blind, randomized, parallel-group study. Oncology 51:113–118
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100(10):2261–2268 May 15
Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Bohidar N, Klinger G, Hustad CM, Horgan KJ, Skobieranda F (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23(12):2822–2830 Apr 20
Weinstein MC (2006) Recent developments in Decision-Analytic Modelling for Economic Evaluation. PharmacoEconomics 24(4):1043–1053
Dranitsaris G, Leung P (2004) Using decision modeling to determine pricing of new pharmaceuticals: the case of neurokinin-1 receptor antagonist antiemetics for cancer chemotherapy. Int J Technol Assess Health Care 20(3):289–295 Summer
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Susan Groshen, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG (1999) Recommendations for the Use of Antiemetics: Evidence-Based, Clinical Practice Guidelines. JCO 2971, Sep 1
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24(18):2932–2947 Epub 2006 May, Jun 20
Cancer Care Ontario: Use of 5-HT3 receptor antagonists in patients receiving moderately or highly emetogenic chemotherapy. Toronto, 1999
Geling O, Eichler HG. Should 5-Hydroxytryptamine-3 Receptor Antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. JCO, 2005, Feb, 23, 6
Uyl-de Groot CA, Wait S, Buijt I (2000) Economics and health-related quality of life in antiemetic therapy: recommendations for trial design. Eur J Cancer 36(12):1522–1535 Aug
Lachaine J, Laurier C (2002) Cost-efficacy analysis of ondansetron regimens for control of emesis induced by noncisplatin, moderately emetogenic chemotherapy. Am J Health Syst Pharm 59(19):1837–1846 Oct 1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Annemans, L., Strens, D., Lox, E. et al. Cost-effectiveness analysis of aprepitant in the prevention of chemotherapy-induced nausea and vomiting in Belgium. Support Care Cancer 16, 905–915 (2008). https://doi.org/10.1007/s00520-007-0349-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0349-1