Abstract
Purpose
To assess the methodological quality of Orphan Medicinal Product (OMP) dossiers and discuss possible reasons for the small number of products licensed.
Methods
Information about orphan drug designation, approval, refusal or withdrawal was obtained from the website of the European Medicines Agency and from the European Public Assessment Reports.
Results
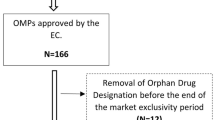
From 2000 up to 2010, 80.9 % of the 845 candidate orphan drug designations received a positive opinion from the European Medicines Agency (EMA)’s Committee on Orphan Medicinal Products. Of the 108 OMP marketing authorizations applied for, 63 were granted. Randomised clinical trials were done for 38 OMPs and placebo was used as comparator for nearly half the licensed drugs. One third of the OMPs were tested in trials involving fewer than 100 patients and more than half in trials with 100–200 cases. The clinical trials lasted less than one year for 42.9 % of the approved OMPs.
Conclusion
Although there may have been some small improvements over time in the methods for developing OMPs, in our opinion, the number of patients studied, the use of placebo as control, the type of outcome measure and the follow-up have often been inadequate. The present system should be changed to find better ways of fostering the development of effective and sustainable treatments for patients with orphan diseases. Public funds supporting independent clinical research on OMPs could bridge the gap between designation and approval. More stringent criteria to assess OMPs’ efficacy and cost/effectiveness would improve the clinical value and the affordability of products allowed onto the market.
Similar content being viewed by others
References
Orphanet. The portal for rare diseases and orphan drugs. Available at: http://www.orpha.net/consor/cgi-bin/index.php?lng=EN. Accessed 19.07.2012
Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. (2000) Off J Eur Communities. 22.01.2000. L18: 1–5
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2001L0083:20070126:en:PDF Accessed 19.07.2012
European Medicines Agency. Rare disease (orphan) designations. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/orphan_search.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d12b Accessed 19.07.2012
European Medicines Agency Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124. Accessed 19.07.2012
Joppi R, Bertele’ V, Garattini S (2006) Orphan drug development is progressing too slowly. Br J Clin Pharmacol 61(3):355–360
Joppi R, Bertele’ V, Garattini S (2009) Orphan drug development is not taking off. Br J Clin Pharmacol 67:494–502
Committee for Proprietary Medicinal Products. Note for guidance on duration of chronic toxicity testing in animals (rodent and non rodent toxicity testing). May (1999). Available at: http://www.emea.europa.eu/pdfs/human/ich/ 030095en.pdf Accessed 19.07.2012
Committee for Medicinal Products for human use. ICH guideline S6 (R1)—preclinical safety evaluation of biotechnology-derived pharmaceuticals. March (1998). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002828.pdf. Accessed 19.07.2012
Keogh M, Sedehizadeh S, Maddison P. Treatment for Lambert-Eaton myasthenic syndrome. The Cochrane Collaboration (2011) Issue 2. Available at: http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD003279/frame.html Accessed 19.07.2012
Anonymous. Treatment for pulmonary arterial hypertension. DynaMed. Available at: http://web.ebscohost.com/dynamed/detail?vid=7&hid=123&sid=1edbca12-c7a5-4663-8308-fa9135beb6de%40sessionmgr113&bdata=JnNpdGU9ZHluYW1lZC1saXZlJnNjb3BlPXNpdGU%3d#db=dme&AN=115043 Accessed 19.07.2012
Anonymous. Treatment for immune thrombocytopenia. DynaMed. Available at: http://web.ebscohost.com/dynamed/detail?vid=9&hid=123&sid=1edbca12-c7a5-4663-8308-fa9135beb6de%40sessionmgr113&bdata=JnNpdGU9ZHluYW1lZC1saXZlJnNjb3BlPXNpdGU%3d#db=dme&AN=114263 Accessed 19.07.2012
Kubota T, Koike R (2010) Cryopyrin-associated periodic syndromes: background and therapeutics. Mod Rheumatol 20:213–221
Hawkes N, Cohen D (2010) What makes an orphan drug? BMJ 341:c6459
http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphanProductDesignation/ucm216147.htm Accessed 19.07.2012
Bashaw ED, Fang L (2012) Clinical pharmacology and orphan drugs: an informational inventory 2006–2010. Clin Pharmacol Ther 91:932–936
Thorat C, Xu K, Freeman SN et al (2012) What the Orphan Drug Act has done lately for children with rare diseases: a 10-year analysis. Pediatrics 129:516–521
European Commission. Communication from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions on rare diseases: Europe’s challenges. 2008 Available at: http://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf Accessed 19.07.2012
Dear JW, Lilitkarntakul P, Webb DJ (2006) Are rare diseases still orphans or happily adopted? The challenges of developing and using orphan medicinal products. Br J Clin Pharmacol 62:264–271
Garattini S (2012) Time to revisit the orphan drug law. Eur J Clin Pharmacol 68(2):113
Acknowledgment
We thank J.D. Baggott for editing.
Conflict of interests
We have no conflict of interests that may be relevant to this work.
Funding
This study was supported by internal institutional funds from the Mario Negri Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joppi, R., Bertele’, V. & Garattini, S. Orphan drugs, orphan diseases. The first decade of orphan drug legislation in the EU. Eur J Clin Pharmacol 69, 1009–1024 (2013). https://doi.org/10.1007/s00228-012-1423-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1423-2