Abstract
Objective
The aim of the study was to describe the prevalence and utilization patterns of methylphenidate (MPH) in children and adolescents in France.
Methods
This was a population-based retrospective study in which the cohort consisted of patients for whom data were extracted from the dispensation drug claims database of the national health insurance (NHI) fund for self-employed workers. Annual prevalence of MPH use was evaluated on patients aged 6–18 years who were reimbursed for at least one MPH prescription a year. Between January 2004 and June 2005, features of MPH medication and user profile were described for the “new starters” having a screening period of 1 year without receiving a MPH prescription and a follow-up ≥12 months. Time to interruption of MPH regular use was analysed by Kaplan-Meier survival analysis. Mean duration of exposure to MPH treatment was computed with the 95% confidence interval (CI).
Results
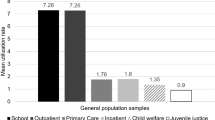
Annual prevalence of MPH per 1000 persons was 1.1 in 2003, 1.5 in 2004 and 1.8 in 2005 (relative increase of 63.5%). New starters (n = 447) received their first MPH prescription through the hospital (65.1%) or through private practitioners (34.9%). The user profiles were: short (16.6%), occasional (33.8%) and regular (49.6%). Among the new starters, the median time to interruption of MPH regular use was 10.2 months (95% CI: 7.9–12.4). The mean duration of exposure to MPH treatment was: occasional (4.9 months, 95% CI: 4.3–5.5) and regular (25.7 months, 95% CI: 24.6–26.8).
Conclusion
Although there is a low prevalence of MPH use in France, this survey revealed a wide profile of users and heterogeneous use patterns.


Similar content being viewed by others
Abbreviations
- ADHD:
-
Attention-deficit/hyperactivity disorder
- MPH:
-
Methylphenidate
- NHI:
-
National health insurance
- RSI:
-
Régime social des indépendants
- SPC:
-
Summary of product characteristics
References
Wong ICK, Murray ML, Camilleri-Novak D, Stephens P (2004) Increased prescribing trends of paediatric psychotropic medications. Arch Dis Child 89:1131–1132
Bonati M, Clavenna A (2005) The epidemiology of psychotropic drug use in children and adolescents. Int Rev Psychiatry 17:181–188
Scheffler RM, Hinshaw SP, Modrek S, Levine P (2007) The global market for ADHD medications. Health Affairs 26:450–457
National Institute for Health and Clinical Excellence NICE Technology (Appraisal 98. Issue March 2006). Methylphenidate, atomoxetine and dexamfetamine for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Available at: http://www.nice.org.uk
Safer DJ, Malever M (2000) Stimulant treatment in Maryland public schools. Pediatrics 106:533–539
Miller AR, Lalonde CE, McGrail KM, Amstrong RW (2001) Prescription of methylphenidate to children and youth, 1990–1996. Can Med Assoc J 165:1489–1494
Montandon JB, Medioni L (2002) Swissmedic. Evolution du nombre de prescriptions de RITALINE (methylphenidate) dans le canton de Neuchâtel entre 1996 et 2000. Available at: http://www.swissmedic.ch
Schmidt-Troschke SO, Ostermann T, Melcher D, Schuster R, Erben CM, Matthiessen PF (2004) The use of methylphenidate in children: analysis of prescription usage based in routine data of the statutory health insurance bodies concerning drug prescriptions. Gesundheitswesen 66:387–392
Vinker S, Vinker R, Elhayany A (2006) Prevalence of methylphenidate use among Israeli children 1998–2004. Clin Drug Invest 26:161–167
Wong ICK, Murray ML (2005) The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol 59:750–755
Faber A, Kalverdijk LJ, de Jong-van den Berg LTW, Hugtenburg JG, Minderaa RB, Tobi H (2006) Parents report on stimulant-treated children in the Netherlands: initiation of treatment and follow-up care. J Child Adolesc Psychopharmacol 16:432–440
Faber A, de Jong-van den Berg LTW, van den Berg PB, Tobi H (2005) Psychotropic co-medication among stimulant-treated children in The Netherlands. J Child Adolesc Psychopharmacol 15:38–43
Ambrosini PJ, Sallee FR, Lopez FA, Shi L, Michaels MA (2006) A community assessment open-label study of the safety, tolerability and effectiveness of mixed amphetamine salts extended release in school-age children with ADHD. Curr Med Res Opin 22:427–440
Lipkin PH, Cozen MA, Thompson RE, Mostofsky SH (2005) Stimulant dosage and age, race and insurance type in a sample of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 15:240–248
Auleley GR, Dematons MN, Berchery P, Raynal-Minville F, Suarez F, Heuls-Brnin B, Blum-Boisgard C (2002) Type 2 diabetes mellitus among beneficiairies of the french national health insurance for self-employed workers (AMPI): comparison of the management of craftsmen or tradesmen with professionals patients. Diabetes Metab 28:491–498
Auleley GR, Deligne J, Hantson C, Blum-Boisgard C (2005) Selective cyclooxygenase-2 inhibitors. A population-based analysis of use in France over a three-year period and comparison with randomised clinical trials. Presse Med 34:703–710
Deligne J, Grimaldi L, Jonville-Béra AP, Giraudeau B, Blum-Boisgard C, Autret-Leca E (2007) Antipyretic drug use in French office based medical practice. Pharmacoepidemiol Drug Saf 16:812–817
Murray ML, Thompson M, Santosh PJ, Wong ICK (2005) Effects of the committee on safety of medicines advice on antidepressant prescribing to children and adolescents in the UK. Drug Safety 28:1151–1157
Institut National de la Statistique et des Etudes Economiques. INSEE (2005) Bilan demographique 2005. Available at: http://www.insee.fr/fr/ffc/pop_age2.htm
Kaplan EL, Meier P (1958) Non-parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Frances C, Hoizey G, Millart H, Trenque T (2002) Methylphenidate (Ritalin®) use in France. Therapie 57:189–193
Frances C, Hoizey G, Millart H, Trenque T (2004) Paediatric methylphenidate (Ritalin) restrictive conditions of prescription in France. Br J Clin Pharmacol 57:115–116
Knellwolf AL, Panei P, Arcieri R, Vella S (2006) Failure to diagnose ADHD correctly puts children in danger. Ital J Pediatr 32:136–137
MTA Cooperative Group (1999) A 14 month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Multimodal treatment study of children with ADHD. Arch Gen Psychiatry 56:1073–1086
Taylor E, Döpfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Sonuga-Barke E, Steinhausen H-C, Zuddas A (2004) European clinical guidelines for hyperkinetic disorder-first upgrade. Eur Child Adolesc Psychiatry 13:1–30
Bonati M (2006) Critical New Perspectives on ADHD. The Italian saga of ADHD and its treatment. In: Lloyd G, Stead J, Cohen (eds) 1st edn, vol 8. Routledge Taylor & Francis Group, Philadelphia, pp 128–136
Centres for Disease Control and Prevention (CDC) (2003) Mental health in the United States: prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder. CDC–MMWR Weekly 54:842–847 (2005). Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5434a2.htm
Skounti M, Philalithis A, Galanakis E (2007) Variations in prevalence of attention deficit hyperactivity disorder worldwide. Eur J Pediatr 166:117–123
Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS (2007) Prevalence, recognition and treatment of attention-deficit/hyperactivity disorder in national sample of US children. Arch Pediatr Adolesc med 161:857–864
Fombonne E (2005) Epidemiology of psychiatric disorders paediatric psychiatry. EMC-Psychiatrie 2:169–194
Direction Generale de la sante/INSERM (2006): Narcolepsie, Cataplexie, Maladie de Gelineau. Orphanet base de donnees sur les maladies rares et les medicaments orphelins. Available at: http://www.orpha.net
Expertise collective INSERM (2002) Mental disorders. Children and adolescents screening and prevention. Institut National de la Sante et de la Recherche Medicale. Available at: http://www.inserm.fr
American Academy of Pediatrics (2001) Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics 108:1033–1044
Barbaresi W, Katusic S, Colligan R, Weaver A, Pankratz V, Mrazek D, Jacobsen S (2004) How common is attention-deficit/hyperactivity disorder? Towards resolution of controversy: results from a population-based study. Acta Paediatr 445:55–59
Acknowledgements
This study was supported by the Italian Medicines Agency (AIFA) within the independent drug research program, contract no. FARM5AJL82. Thanks to Romano Arcieri (National Institute of Health, Italy), Maurizio Bonati (Mario Negri Pharmacological Research Institute, Italy) and Catherine Billard (Kremlin Bicètre Hospital, France) for reviewing the manuscript, to Ernesto Costabile (National Institute of Health, Italy) for support in the literature review, to Maria Grazia Caparra (external consultant) for English revision.
Conflict of interest statement
None of the authors have competing interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Copyright and other legal and ethical requirements.
The authors guarantee that the manuscript will not be published elsewhere in any language without the consent of the copyright owners.
Rights and permissions
About this article
Cite this article
Knellwolf, AL., Deligne, J., Chiarotti, F. et al. Prevalence and patterns of methylphenidate use in French children and adolescents. Eur J Clin Pharmacol 64, 311–317 (2008). https://doi.org/10.1007/s00228-007-0401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0401-6