Abstract
Summary
Automatic generic substitution of alendronate products, used to reduce drug costs, and medication persistence was studied retrospectively between 2006 and 2009. During this period the number of, and the rate of substitution between, alendronate products increased while persistence decreased. Patient preferences should be considered when designing and evaluating generic policies.
Introduction
Automatic generic substitution (AGS) was implemented in Sweden in 2002. The objective of this study was to investigate the association between AGS and persistence with alendronate treatment of primary osteoporosis in Sweden.
Methods
An open historical cohort of women and men (n = 36,433) was identified in the Swedish Prescribed Drug Register through filled prescriptions for alendronate or risedronate between 2005 and 2009. Co-morbidity data was extracted from the National Patient Register. The association between AGS and medication persistence was investigated using non-parametric and parametric survival analysis.
Results
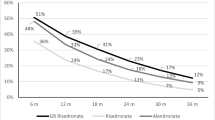
Between 2006 and 2009, the number of alendronate products increased from 15 to 25, the proportion of prescriptions constituting a substitution increased from 10.8% to 45.2%, and the proportion of patients persisting with alendronate treatment for 12 months fell from 66.9% to 51.7%. Patients starting alendronate treatment in 2006 had lower risk of stopping treatment compared with those starting in 2007 (HR 1.34, 95% CI 1.29–1.39), 2008 (HR 1.49, 95% CI 1.43–1.55), and 2009 (HR 1.50, 95% CI 1.40–1.60). No difference was observed in persistence with proprietary risedronate during the same period. Individuals who had their alendronate product substituted at the first prescription refill had significantly higher probability of discontinuation (HR 1.25, 95% CI 1.20–1.30).
Conclusion
AGS causes increased product substitution which appears to be associated with reduced treatment persistence. Poor health outcomes and associated costs due to forgone drug exposure should be taken into account in the design and evaluation of policies implemented to encourage utilisation of generic medicines.




Similar content being viewed by others
References
OECD, Statistics from A to Z (available from: http://www.oecd.org/document/0,3746,en_2649_201185_46462759_1_1_1_1,00.html (accessed June 20, 2011)).
Aalto-Setala V (2008) The impact of generic substitution on price competition in Finland. Eur J Health Econ 9(2):185–91
Andersson KA, Petzold MG, Allebeck P, Carlsten A (2008) Influence of mandatory generic substitution on pharmaceutical sales patterns: a national study over five years. BMC Health Serv Res 8:50
Sander JW, Ryvlin P, Stefan H, Booth DR, Bauer J (2010) Generic substitution of antiepileptic drugs. Expert Rev Neurother 10(12):1887–98
WHO, Adhence to long term therapies: evidence for action. 2003 (Available from: http://whqlibdoc.who.int/publications/2003/9241545992.pdf accessed March 5, 2010).
Strom O, Borgstrom F, Kanis JA, Jonsson B (2009) Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int 20(1):23–34
Socialstyrelsen, Patientsäkerhet vid utbyte av läkemedel på apotek. (Available from: http://www.socialstyrelsen.se/publikationer2004/2004-103-14 (accessed June 10, 2011)).
Duerden MG, Hughes DA (2010) Generic and therapeutic substitutions in the UK: are they a good thing? Br J Clin Pharmacol 70(3):335–41
Heikkila R, Mantyselka P, Ahonen R (2011) Do people regard cheaper medicines effective? Population survey on public opinion of generic substitution in Finland. Pharmacoepidemiol Drug Saf 20(2):185–91
The Swedish Medical Products Agency (2007) behandling av osteoporos 18:19–38
TLV, The Dental and Pharmaceutical Benefits Agency. (Available from: http://www.tlv.se accessed April 8, 2011).
SCB, Statistics Sweden. (Available from: http://www.scb.se accessed April 10, 2011).
Socialstyrelsen (Available from: http://www.socialstyrelsen.se/register accessed April 23, 2011).
Landfeldt, E., O. Strom, S. Robbins, and F. Borgstrom, Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int, 2011.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–9
TLV, The Dental and Pharmaceutical Benefits Agency: Översyn av 2011 års modell för utbyte av läkemedel på apotek. 2011 (Available from: www.tlv.se accessed Oct 5, 2011).
Landfeldt E, Lundkvist J, Strom O (2011) The societal burden of poor persistence to treatment of osteoporosis in Sweden. Bone 48(2):380–8
Blouin J, Dragomir A, Ste-Marie LG, Fernandes JC, Perreault S (2007) Discontinuation of antiresorptive therapies: a comparison between 1998–2001 and 2002–2004 among osteoporotic women. J Clin Endocrinol Metab 92(3):887–94
Sheehy O, Kindundu CM, Barbeau M, LeLorier J (2009) Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int 20(8):1369–76
Cramer JA, Lynch NO, Gaudin AF, Walker M, Cowell W (2006) The effect of dosing frequency on compliance and persistence with bisphosphonate therapy in postmenopausal women: a comparison of studies in the United States, the United Kingdom, and France. Clin Ther 28(10):1686–94
Strom, O., F. Borgstrom, J. Kanis, J. Compston, C. Cooper, E. McCloskey, and B. Jonsson, Osteoporosis: burden, health care provision and opportunities in the European Union. Archives of osteoporosis, 2011. E-pub ahead of print.
European Commission. Analysis of differences and commonalities in pricing and reimbursement systems in Europe. (Available from: http://www.easp.es/web/Documentos/OtrosDocumentos/EASPReportPandREurope.pdf accessed July 2, 2011).
Himmel W, Simmenroth-Nayda A, Niebling W, Ledig T, Jansen RD, Kochen MM, Gleiter CH, Hummers-Pradier E (2005) What do primary care patients think about generic drugs? Int J Clin Pharmacol Ther 43(10):472–9
Horne R, Weinman J (1999) Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 47(6):555–67
Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK (2011) Physician perceptions about generic drugs. Ann Pharmacother 45(1):31–8
Andersson K, Jorgensen T, Carlsten A (2006) Physicians' opinions and experiences of the Pharmaceutical Benefits Reform. Scand J Public Health 34(6):654–9
Shakweh M, Bravo-Osuna I, Ponchel G (2007) Comparative in vitro study of oesophageal adhesiveness of different commercial formulations containing alendronate. Eur J Pharm Sci 31(5):262–70
Grima DT, Papaioannou A, Airia P, Ioannidis G, Adachi JD (2010) Adverse events, bone mineral density and discontinuation associated with generic alendronate among postmenopausal women previously tolerant of brand alendronate: a retrospective cohort study. BMC Musculoskelet Disord 11:68
Halkin H, Dushenat M, Silverman B, Shalev V, Loebstein R, Lomnicky Y, Friedman N (2007) Brand versus generic alendronate: gastrointestinal effects measured by resource utilization. Ann Pharmacother 41(1):29–34
Conflicts of interest
Both authors have previously consulted for companies marketing products for osteoporosis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ström, O., Landfeldt, E. The association between automatic generic substitution and treatment persistence with oral bisphosphonates. Osteoporos Int 23, 2201–2209 (2012). https://doi.org/10.1007/s00198-011-1850-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1850-4