Abstract
The objective of this work was to examine the prediction of incident hip fracture risk by composite indices of femoral neck strength, constructed from dual X-ray absorptiometry (DXA) scans of the hip. These indices integrate femoral neck size and body size with bone density, and reflect the structure’s ability to withstand axial compressive forces and bending forces, and the ability to absorb energy in an impact. We studied a prospective cohort of community-dwelling women, 55 years or older at baseline, average age 72 years, that was established in 1988, with follow-up until 1998. Higher baseline values of each composite strength index were associated with lower risk of incident hip fracture in this cohort. After adjusting for age and body mass index, relative risks associated with each standard deviation increment in the composite indices were 0.39 for compression strength (95% confidence interval 0.25–0.60), 0.43 for bending strength (95% confidence interval 0.27–0.70), and 0.34 for impact strength (95% confidence interval 0.20–0.58). Larger bone size relative to body size was associated with lower risk of incident hip fracture. After adjusting for age, body mass index, and bone density, each standard deviation increment in femoral neck width normalized by body weight was associated with incident hip fracture relative risk of 0.38 (95% confidence interval 0.20–0.70). Our results suggest that composite indices of femoral neck strength, constructed from DXA measurements, have the potential to improve hip fracture risk assessment. This needs further exploration in larger cohorts and in different population sub-groups.


Similar content being viewed by others
References
Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM for The SOF Research Group (1995) Risk factors for hip fractures in white women. N Engl J Med 332: 767–773
Vose GP, Lockwood RM (1965) Femoral neck fracturing—its relationship to radiographic bone density. J Gerontol 20:300–305
Allolio B (1999) Risk factors for hip fracture not related to bone mass and their therapeutic implications. Osteoporos Int 10:S9–S16
Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK (1993) Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res 8:1211–1217
Cheng XG, Lowet G, Boonen S, Nicholson PH, Brys P, Nijs J, Dequeker J (1997) Assessment of the strength of proximal femur in vitro: relationship to femoral bone mineral density and femoral geometry. Bone 20:213–218
Dalen N, Hellstrom LG, Jacobson B (1976) Bone mineral content and mechanical strength of the femoral neck. Acta Orthop Scand 47:503–508
Cordey J, Schneider M, Belendez C, Ziegler WJ, Rahn BA, Perren SM (1992) Effect of bone size, not density, on the stiffness of the proximal part of normal and osteoporotic human femora. J Bone Miner Res 7:S437–S444
Ismail AA, O’Neill TW, Cockerill W, Finn JD, Cannata JB, Hoszowski K, Johnell O, Matthis C, Raspe H, Raspe A, Reeve J, Silman AJ (2000) Validity of self-reports of fractures: results from a prospective study in men and women across Europe. Osteoporos Int 11:248–254
Joakimsen RM, Fonnebo, Sogaard AJ, Tollan A, Stormer J, Magnus JH (2001) The Tromso Study: registration of fractures, how good are self reports, a computerized radiographic register, and discharge reegister? Osteoporos Int 12:1001–1005
Hayes WC, Myers ER, Morris JN, Gerhart TN, Yett HS, Lipsitz LA (1993) Impact near the hip dominates fracture risk in elderly nursing home residents who fall. Calcif Tissue Int 52:192–198
Robinovitch SN, Hayes WC, McMahon TA (1991) Prediction of femoral impact forces in falls on the hip. Trans ASME 113:366–374
Levy PS, Lemeshow S (1980) Nonresponse and missing data in sample surveys. In: Levy PS (ed) Sampling for health professionals. Lifetime Learning Publications, Belmont, Calif., pp 258–276
Parsons VL (2000) Design and estimation structures for the 1995–2004 NHIS. In: Design and estimation for the National Health Interview Survey, 1995–2004. National Center for Health Statistics, pp 14–23. [Vital and Health Statistics, Series 2(130)]
Seeman E, Duan Y, Fong C, Edmonds J (2001) Fracture site specific deficits in bone size and volumetric density in men with spine or hip fractures. J Bone Miner Res 16:120–127
Alonso CG, Curiel MD, Carranza FH, Cano RP, Perez AD, and the Multicenter Project for Research in Osteoporosis (2000) Femoral bone mineral density, neck-shaft angle and mean femoral neck width as predictors of hip fracture in men and women. Osteoporos Int 11:714–720
Ensrud KE, Lipschutz RC, Cauley JA et al. (1997) for the Study of Osteoporotic Fractures Research Group. Body size and hip fracture risk in older women: a prospective study. Am J Med 103:274–280
Bergot C, Bousson V, Meunier A et al. (2002) Hip fracture risk and proximal femur geometry from DXA scans. Osteoporos Int 13:542–550
Dretakis EK, Papakitsou E, Kontakis GM et al. (1999) Bone mineral density, body mass index, and hip axis length in postmenopausal Cretan women with cervical and trochanteric fractures. Calcif Tissue Int 64:257–258
Lochmuller EM, Miller P, Burklein D, Wehr U, Rambeck W, Eckstein F (2000) In situ femoral dual-energy X-ray absorptopmetry related to ash weight, bone size and density, and its relationship with mechanical failure loads of the proximal femur. Osteoporos Int 11:361–367
Smith CB, Smith DA (1976) Relations between age, mineral density, and mechanical properties of human femoral compacta. Acta Orthop Scand 47:496–502
Beck TJ, Ruff CB, Warden KE, Scott Jr WW, Rao GU (1990) Predicting femoral neck strength from bone mineral data: a structural approach. Invest Radiol 25:6–18
Klenerman L, Swanson SAV, Freeman MAR (1967) A method for the clinical estimation of the strength of a bone. Proc R Soc Med 60:10–14
Beer FP, Johnston Jr ER (1992) Mechanics of materials, 2nd edn. McGraw Hill, New York
Martin RB (1991) Determinants of mechanical properties of bones. J Biomech 24 (suppl 1):79–88
Martin RB, Burr DB (1984) Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech 17:195–201
Smith RW Jr, Walker RR (1964) Femoral expansion in aging women: implications for osteoporosis and fractures. Science 145:156–157
Acknowledgements
Work on this article was supported by NIH/NIA Mentored Clinical Scientist Development Award 1K12AG01004. The authors would like to gratefully acknowledge the assistance of Gary Beaupre and Dana Carpenter of the Department of Mechanical Engineering, Stanford University, Stanford, Calif., USA, for their careful reading of the manuscript and many suggestions. The authors would also like to acknowledge the contributions of anonymous reviewers whose suggestions greatly improved the presentation of the development of the risk scores.
Author information
Authors and Affiliations
Appendix: derivation of composite strength indices
Appendix: derivation of composite strength indices
Here, we derive composite indices of femoral neck strength using structural engineering principles. We look at the femoral neck’s ability to resist compressive loads (along its main axis) and bending loads, and its ability to absorb energy in an impact.
Some fractures of the femoral neck may be the result of compressive forces along the femoral neck axis. Basic concepts from strength of engineering materials show that if material properties are constant within a structure, then the ability of the structure to withstand axial compressive forces at any point is proportional to its cross-sectional area at that point. For instance, the compressive force needed to create unit axial strain is given by [23]:
Similarly, if the ultimate axial stress that the material can tolerate in the elastic mode (material strength) is σlim, then the compressive force needed to cause failureis given by [24]:
Young’s elastic modulus and material strength of bone are material properties that depend both on the quantity (mass) of mineral and on the distribution of mineral within bone [24]. Material properties are generally not uniform within whole bone, and differ for instance, between trabecular and cortical regions. Since details of bone composition are not readily available from DXA scans, we assume here that mineral mass in the femoral neck is largely in the cortex. This permits us to use macroscopic measurements of bone mass and size (from DXA) to gauge strength.
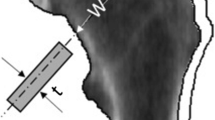
With this simplifying assumption, the femoral neck’s compressive strength (ability to withstand an axial compressive load) is proportional to the cross-sectional area of the mineralized cortex at its thinnest point, a purely structural factor. Although the cortex’s cross-sectional area is not directly obtainable from a DXA scan, it is related to the areal bone mineral density measured by DXA because of the following equality:
where ρ is the cortex’s effective density (in g/cc), CCSA is the cortical cross-sectional area (in cm2), BMD is the projected areal bone density measured by DXA (in g/cm2) and FNW is the width of the femoral neck at its narrowest point in the DXA projection plane (in cm). This equality follows because the product on either side equals the total bone mineral content per unit-thickness cross-sectional slice (in g/cm). Although apparent density of whole bone (bone mass divided by whole bone volume) varies from person to person because of differences in porosity and soft tissue elements, the actual density of mineralized bone tissue varies relatively little, even in osteoporosis [21,25]. Since ρ is essentially constant, CCSA is directly proportional to BMD*FNW. Hence, the following potential measure of femoral neck compressive strength can be constructed from DXA scans:
With respect to fracture risk, absolute strength is not as important as strength relative to the size of the load: Structures that are exposed to larger loads need to be stronger than structures exposed to less heavy loads. The load borne by the femur during walking is proportional to body weight. Hence, we have the following composite index of the femoral neck’s compression strength, relative to its usual load:
Some fractures of the femoral neck may be a result of bending forces. Again, basic concepts from strength of engineering materials show that if material properties are constant in a structure, then its ability to withstand a bending moment at any point is proportional to its section modulus at that point [23]. For instance, the bending force needed to create unit axial strain in the outermost fibers (where the strain is maximum) is given by:
Similarly, if material strength is σlim, then the bending force needed to cause failure in the outermost fibers is given by:
While material strength and elastic modulus are both material properties, the second term that is common to both of the above expressions, the ratio of section modulus to moment arm, is a structural factor [24]. The section modulus of a structure at any point depends on the distribution of mass in its cross section at that point. Determining it exactly for a bone structure such as the femoral neck requires knowledge of the distribution of bone mineral in the femoral neck, which may be obtained either from three-dimensional computed tomography data or from the absorption profile along the neck width from either single photon absorptiometry [25] or DXA [21]. In the absence of such detailed information, the section modulus of the femoral neck can be estimated in the following fashion. Assuming that the cross-sectional shape of the femoral neck is approximately circular and that mineral mass is largely confined to its cortical rim, it follows that
where R and r are the outer and inner radii of the mineralized annular cortex in cross section [26]. Notice that the first term in the above expression is the cross-sectional area of the mineralized cortical annulus. In the absence of direct measurement of the inner radius r, a crude approximation to the second term, which is appropriate for thin cortices, is obtained by using half of FNW for both R and r, so that the section modulus of the femoral neck at its thinnest point can be approximated by:
The earlier discussion showed that CCSA is proportional to BMD*FNW; therefore the section modulus of the femoral neck is proportional to BMD*FNW*FNW. Using this formula for section modulus, and using HAL as the moment arm, we obtain the following measure of the femoral neck’s bending strength (ability to withstand a bending load):
This is an absolute measure of bending strength. Since the bending forces borne by the femur during walking are proportional to body weight, we have the following composite index of the femoral neck’s bending strength, relative to its usual load:
However, the vast majority of hip fractures in older men and women occur as a result of a fall [10]. The forces experienced in a fall depend not only on the weight of the person but also on the height of the fall [23]. In fact, because of the proportionality of individual body parts to overall body size, the potential energy to be absorbed in a fall from standing height is proportional to the product of body height and body weight.
It can be shown that the maximum energy that a cylindrical structure (such as the femoral neck) can absorb in axial impact before stresses reach the elastic limit σlim, is given by [23]:
where Y is the Young elastic modulus of the material. Similarly, the maximum energy that a cylindrical structure with annular cross-section can absorb in bending mode before stresses reach the elastic limit σlim, is given by [23]:
Recalling that for the femoral neck, R equals FNW/2 and the section modulus is proportional CCSA*FNW, and using HAL for both the length of the structure and the moment arm, it follows that the maximum energy that the femoral neck can absorb in both compressive and bending modes is proportional to the same structural factor: CCSA*HAL. As before, CCSA can be estimated from DXA data as the product of BMD and FNW. Thus, the following potential measure of femoral neck (absolute) impact strength can be constructed from DXA scans:
Since the potential energy in a fall from standing height is proportional to the product of body height and weight, we have the following composite index of the femoral neck’s impact strength, relative to the energy in a fall from standing height:
Rights and permissions
About this article
Cite this article
Karlamangla, A.S., Barrett-Connor, E., Young, J. et al. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int 15, 62–70 (2004). https://doi.org/10.1007/s00198-003-1513-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-003-1513-1