Abstract
Purpose
In 2005 the International Committee of Medical Journal Editors issued a requirement that all randomized controlled trials (RCTs) be registered primarily to prevent selective reporting (publication bias). However, registries allow for alterations in study protocol. Changes occurring before (or after) study completion could invalidate the original study intent, leading to publication of misleading conclusions. In RCTs involving critically ill patients, these concerns may be particularly acute because mortality is high and conditions investigated are usually syndromes rather than specific diseases. This study was conducted to estimate the registration rate of RCTs in critical care; and, among registered RCTs, to determine timing of registration and whether sample size or primary outcome were altered.
Methods
We searched the MEDLINE database for RCTs that began after or continued through July 2005. We determined whether each trial had been registered and, for registered trials, compared registry data to data in the published manuscript.
Results
Approximately two-thirds (66 %) of trials were registered. Of these, 66 % of registrations occurred after enrolment had commenced. Overall, 6 % (5/90) of trials appropriately registered a sample size which was unchanged from the interval between registration and publication, and only 12 % (11/90) reported primary outcomes that were both appropriately registered and unchanged.
Conclusions
Non-registration, or registration after trial initiation, are common in RCTs of critically ill patients. Among registered trials important protocol changes are often made between trial commencement and publication. This study identifies and quantifies the extent of this serious—but correctable—problem for RCTs in critically ill patients.
Similar content being viewed by others
Introduction
The randomized controlled trial (RCT) is preferred for testing cause–effect relationships between treatments and outcomes [1] but its validity depends upon several important elements. Two important components of an RCT are the primary outcome—the principle issue being tested—and the anticipated sample size. A larger sample size is required in studies where the between-group difference in primary outcome (i.e. effect size) is small, or where its variance is large; thus these two key elements are interrelated [2].
Changing a primary endpoint after enrolment has begun changes the principle purpose of the research; it also changes the original assumptions that were used to calculate the required sample size. Similarly, changing the sample size while the trial is underway suggests an inability to enrol adequate numbers of patients, or that the anticipated effect size was incorrect. Nonetheless, while changing the primary endpoint or sample size after enrolment has commenced can undermine the validity of a study, transparent reporting facilitates detection and understanding of such changes.
Recognizing such concerns, the National Institutes of Health (NIH) and other agencies created databases for trial pre-registration. Supporting this initiative, the International Committee of Medical Journal Editors (ICMJE) agreed that RCTs would not be considered for publication unless they had been registered with a minimum dataset [3, 4]. Using such registration databases, journal reviewers and readers can determine the originally recorded intentions of the study and whether (and when) such plans had been changed. In addition, pre-registration enables better identification of publication bias, protecting against the non-publication of ‘negative’ studies [5].
Understanding the timing of trial registration may provide important insight into the significance of alterations in the study plan. Changes that occurred between trial completion and publication may have been prompted by the final results, whereas changes occurring during patient enrolment may have been triggered by the accumulating study data. In contrast, the combination of registration before commencement of patient enrolment coupled with no subsequent design changes (or restriction to well-justified changes) ensures that neither emerging nor completed study data would have influenced the study design as reported in the final manuscript. While the original requirement was for registration before commencement of patient enrolment [3, 4], ‘appropriate’ registration has more recently been characterized as that occurring before completion of the trial [6].
Changes in study design may have particular impact on RCTs of critically ill patients, where the presence of multiple therapies and co-morbidities is common [7–9]. In addition, because of the high mortality, morbidity and economic cost associated with critical illness, it is especially important to design studies with sufficient power to determine with certainty whether therapies investigated in trials are truly effective—or not [10]. Altering the intended outcome during trial conduct can introduce similar barriers to understanding the effectiveness of a tested intervention. These concerns are heightened in the critically ill because a large majority (over 90 %) of multicenter RCTs in critical care medicine (CCM) that specify mortality as a primary endpoint reported that the tested intervention was non-beneficial [11].
Because pre-registration can facilitate insight into the validity of an RCT, and because this may be especially important in the critically ill, we investigated trial registration and post-registration trial alterations in published RCTs of treatments in ICU patients.
Methods
Search and selection criteria
We searched the MEDLINE database (August 2011) using OVID Medline to identify RCTs that were published in the discipline of CCM using the following MeSH terms: “Critical Care”, “Critical Illness”, “Intensive Care Units”, “Respiratory Distress Syndrome, Adult”, “Sepsis”, “Multiple Organ Failure”, and “Respiration, Artificial”. We limited our search to include only trials conducted in humans and published in English-language journals (“English language” and “humans”) and used the validated search term “randomized controlled trials” (pt). We also chose to limit our search to studies published after 2005 (“2005–August 2011”) because most major medical journals required trial registration by this date.
Two reviewers (VA, BK) independently screened studies for inclusion according to the following criteria: trials involving interventions in intensive care units, burn units, or pulmonary care units. Exclusion criteria included completion of enrolment before July 2005; focus on perinatal or neonatal issues, pharmacokinetics, follow-up clinics, caregiver knowledge or validation of scoring systems; secondary analyses of a prior study; retracted manuscripts; studies of pre- or intraoperative interventions; and studies in healthy volunteers or in sleep laboratories.
We searched for trials published since 1 July 2005 because the 2004 ICMJE statement stipulated that trials commencing after this date must be registered at or before the onset of enrolment. However, the ICMJE recognized that trials already commenced prior to this date might not yet have been registered, and required that such ongoing or completed trials be registered before 13 September 2005 to be considered for publication.
The registry identification number was recorded from the manuscript. For published trials that did not include their registry data, a search using the name of the first and last author (and the corresponding author, where this was neither the first nor last author) was conducted in the three most commonly used registration databases: ClinicalTrials.gov (NCT), controlled-trials.com (ISRCTN), and anzctr.org.au (ACTRN). If registration information was not identified, an email was sent to the corresponding author to enquire about registration status.
Manuscript review
Two reviewers independently abstracted information from the published manuscript of each registered trial. The enrolment start and end dates were recorded, if reported. If no enrolment date was reported, enrolment commencement and cessation dates from the registry were used, where available. The primary outcome of the study was recorded; if none was specified, the outcome variable that was used to determine sample size was assumed to be the primary outcome. If the reviewers were still unable to determine a primary outcome, the primary outcome was recorded as ‘unclear’. If more than one primary outcome (or primary efficacy or safety endpoints) were reported, all were recorded. The number of enrolled patients that were included in the final analysis was also recorded.
Registry review
Two reviewers abstracted the following information from the online registry: registration date, primary outcome, anticipated sample size. The date on which the study was registered in the database was recorded, as was the proposed primary outcome(s), as well as any dates that these were entered or altered. If a primary outcome was not recorded at the time of registration, but was subsequently appended, they were reported as ‘added’. The anticipated enrolment size was recorded as present or not.
Evaluation measures
The enrolment start and end dates (from the published paper) were compared to the trial registration date (from the registry). Studies in which enrolment commenced after 1 July 2005 (the date stipulated by the ICMJE statement) were examined for registration date and these dates were recorded as ‘registered before patient enrolment commenced’, ‘registered during patient enrolment’, and ‘registered after patient enrolment was completed’.
For trials that commenced before, but continued after 1 July 2005, registration was recorded as ‘studies registered on or before September 2005’ or ‘studies registered after September 2005’.
Primary outcomes and sample size
Changes in the primary outcome(s) between initial registration and publication in the manuscript primary outcome(s) were recorded; all detected changes were confirmed by three reviewers (BK, DS, CP) independently and then in conference. Consensus was used to resolve any disagreements. We a priori defined as important a difference in sample size of 10 % or more between that originally registered and that reported in the published manuscript.
ICMJE status
For each journal from which a trial was included in the analysis, their listing status with ICMJE was recorded. The source of this information was http://www.icmje.org/journals.html (journals following ICMJE recommendations).
Results
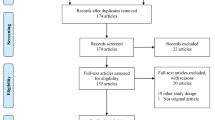
Our search identified 2,308 published studies and 197 trials met our eligibility criteria (Fig. 1). Overall, 133 (of 197; 68 %) trials were registered in a trials registry; 105 reported the registry number in the manuscript and 28 were found by registry searches. Most studies were registered with ClinicalTrials.gov (n = 103) or Controlled-Trials.com (n = 25), and 5 studies were registered with the Australian New Zealand Clinical Trials Registry. One study reported incorrect registration information but was nonetheless included in our analysis. For two studies in which enrolment completion was not recorded in the publication, the completion date from the registry was used. Five studies had incomplete enrolment date information in both publication and registry, and were excluded from further analysis. The included studies were divided into those starting enrolment after July 2005 (n = 90; Fig. 1) vs. those in which enrolment commenced before July 2005 (n = 107; Fig. 1).
Enrolment commenced after July 2005
Registration and timing of registration (“Appendix 2”)
Two-thirds (59/90; 66 %) of trials commencing after July 2005 were registered (Fig. 1). Of the 59 registered trials, 20 (34 %) were registered before patient enrolment began, 23 (39 %) were registered during the enrolment phase of the trial, and 16 (27 %) were registered after enrolment was completed (Fig. 1). Approximately one-third (31/90; 34 %) did not have identifiable registration information; in these cases the corresponding author was contacted, nine of whom confirmed non-registration. No additional registration information was available.
Sample size (“Appendix 3”)
Of the 90 critical care trials included in the study, 5 (6 %) articles were both appropriately registered (registered prior to study enrolment), and had unchanged sample size from registration to publication (Fig. 2). In total 55 % (11/20) of pre-registered trials changed sample size by at least 10 %. Of trials registered during enrolment, 11/23 (48 %) changed the sample by at least 10 %; of these 82 % (9/11) were revised to a lower value. In a further 19 % (11/59) of registered trials the original sample size recorded in the registry was unclear.
Sample size in trials where all enrolment occurred after July 2005: before trial = registration occurring before trial enrolment or “pre-registration”; during trial = registration occurring while trial enrolment was ongoing; after trial = registration occurring after trial enrolment was completed or “post-registration”; not changed = trials where sample size was clearly not altered from registration to publication; changed = trials where sample size was definitely changed from registration to publication; unclear = trials where alteration of sample size was unclear
Primary outcome (“Appendix 4”)
Eleven of 90 (12 %) trials were appropriately registered and unchanged (Fig. 3). In 25 % (15/59) of registered trials a change was made in published primary outcome from that recorded in the registry. In 56 % (33/59) of registered trials a change in primary outcome was unclear either because of the lack of clearly identifiable primary outcome or because registration occurred after the trial enrolment began. Changes to a primary outcome often involved the reporting of mortality (3/7 RCTs registered pre-enrolment and 3/6 registered mid-enrolment) (“Appendix 7”).
Primary outcome in trials where all enrolment occurred after July 2005: before trial = registration occurring before trial enrolment or “pre-registration”; during trial = registration occurring while trial enrolment was ongoing; after trial = registration occurring after trial enrolment was completed or “post-registration”. Not changed = trials where primary outcome was clearly not altered from registration to publication; changed = trials where primary outcome was definitely changed from registration to publication; unclear = trials where alteration of primary outcome was unclear
Enrolment commenced before July 2005
Registration and timing of registration
For 107 studies in which enrolment commenced prior to July 2005 and continued through 13 September 2005, 74 (69 %) were registered; of these almost one-third (35 %, 26/74) were registered after enrolment was complete.
ICMJE status
Comparisons were made between papers published in journals listed as following ICMJE recommendations (‘Listed’) vs. papers published in journals that were not listed as following ICMJE recommendations (‘Non-listed’). Registration was confirmed in 83 % of papers published in ‘Listed’ journals vs. 57 % published in ‘Non-listed’ journals (Table 1; p = 0.019, OR 3.57, 95 % CI 1.09–12.35). Sample size was changed (by ≥10 %) in 30 % of papers published in ‘Listed’ journals vs. 64 % of papers published in ‘Non-listed’ journals (Table 2; p = 0.02, OR 0.24, 95 % CI 0.06–0.95). Finally, primary outcome was changed in 30 % of papers published in ‘Listed’ journals vs. 26 % of papers published in ‘Non-listed’ journals (Table 3. p = 0.73).
Discussion
We systematically evaluated published RCTs in critical care commencing enrolment after July 2005 and were unable to find an associated registration in over one-third. Thus, as in other areas of medicine [6, 12–14], clinical trials conducted in critically ill patients frequently do not conform to the recommendations of the ICMJE statement [3, 4]. Of trials in critically ill that were registered (at any time), registration occurred either during or after completion of patient enrolment in two-thirds of studies. In addition, among trials with significant changes in study design (i.e. sample size or primary outcome) between initial registration and eventual publication, the majority of these changes occurred during or after patient recruitment. Finally, our results likely underestimate the extent of this problem because protocol changes in trials that were not registered until after study completion cannot be detected; in addition while ClinicalTrials.gov allows tracking of protocol changes, other registries are not as easily tracked.
The ICMJE statement on trial registration was intended to minimize publication bias and increase transparency in reporting of trials. Such registration helps protect against selective reporting (and duplication of results) because investigators publicly declare the methodology and study purpose [3, 4], and do not alter these except for sound reasons that should be articulated. Registration helps confirm the study’s internal validity where primary outcomes and sample size remain as determined at the design phase. In contrast, internal validity is undermined if the outcomes or target sample size is shaped by evolving (or final) results. Thus, our finding that less than a fifth of all trials registered were done so before patient enrolment began and clearly did not change the primary outcome between registration and publication suggests that published manuscripts might not accurately represent the original study intent or design.
We found that the anticipated sample size frequently differed between initial registration and manuscript publication; when such discrepancies occurred, the published sample size was usually lower than that initially recorded. Reducing sample size during the conduct of a study may render the study underpowered to detect a clinically important difference between groups [10, 15]. Conversely, studies that report significant between-group differences should be viewed with skepticism where the sample size has been markedly reduced during the course of the study [16]; in these cases, the effect size (i.e. the magnitude of the apparent treatment benefit) is frequently overestimated, as has been described in cases of premature trial termination for apparent benefit [17].
We focused on trials in the critically ill because such studies may be particularly susceptible to the problems created by inadequate trial registration. For example, important heterogeneity exists in many aspects of these patients. Illness definitions—and thus criteria for trial entry—in such patients are usually syndromes (e.g. sepsis, acute respiratory distress syndrome) rather than specific disease entities; as well, management is often multifaceted and co-morbidities are common. Thus, trials in these patients involve much ‘study noise’, making imperative the standardization and consistency of trial management. These factors may explain, in part, why the overwhelming majority (over 90 %) of multicenter RCTs with mortality as a primary endpoint in this population report that the tested interventions were not beneficial [11].
Studies testing mortality as the primary endpoint may be the most important to patients. Because critically ill patients have very high levels of mortality [18], morbidity [19–23] and economic cost [24], design changes that may contribute to incorrect or misleading reports assume a high priority. In addition to such concerns, there are ethical implications: altering the design of a study after consent has been obtained could compromise the nature of the consent [10], and may be especially important in CCM where consent is frequently through a third-party [25]. When changes to primary outcome were recorded, these often involved the reporting of mortality (3/7 RCTs registered pre-enrolment with subsequent changes and 3/6 registered mid-enrolment). We believe this is a conservative number, as several studies had multiple primary endpoints making it unclear if ‘the’ primary endpoint was changed.
Previous reports have raised concerns about the discrepancies between registered and published trial methodologies. Mathieu and colleagues evaluating trials from three different subspecialties (cardiology, rheumatology, and gastroenterology) reported that over 50 % of trials were not ‘adequately’ registered [6], and among these studies, the primary outcome was altered in one-third of trials, almost always (over 80 %) conferring ‘statistical advantage’ towards a positive trial result. However, this may be an underestimate of the problem, as in that report [6] registration was considered to be ‘adequate’ provided it occurred before study completion, thereby missing—in those studies—any design changes that may have been made during patient enrolment.
Other issues can undermine trial registration. For example, a study of trial registration in Canada reported non-compliance with identification of trial leadership and contact information, two (of the 20) important items identified as necessary by the ICMJE [26].
Lack of adherence to the principles outlined in the 2005 ICMJE statement [3, 4] may occur for several reasons, including lack of understanding or acceptance by researchers, inadequate review of registration data by manuscript reviewers, and insufficient oversight from editorial boards. However, in some cases lack of adherence may reflect a desire to change sample size or primary outcome in order to enhance the likelihood of earlier publication, or publication in a higher-impact journal. Our data suggest that attention to the timing of registration, as well as changes in key elements such as sample size and primary outcome, could enhance registry benefit in studies of the critically ill.
There are important limitations to our findings. First, the evaluation focused on trials in CCM, and thus may not reflect the prevalence of inadequate registration in other disciplines. In fact, review of trial registration in other subspecialties (i.e. cardiology, rheumatology, and gastroenterology) has revealed comparable rates registration, although sample size alterations during the study were not sought [6].
The study was limited to a relatively small time span (i.e. 6 years since the publication of the ICMJE statement) [3, 4]. This may be important because trials that have commenced since 2005 might not yet be completed or published, and therefore rates of pre-commencement trial registration might now be greater than reported in this study. However, while the detection of discrepancies between registered and published trial information is facilitated by insistence on pre-commence registration, such changes can still occur. Our analysis of ICMJE status is limited since there may be journals not listed with the ICMJE who follow the guidelines, and conversely there may be some journals listed with the ICMJE who do not follow all of the recommendations. We acknowledge that our search strategy may have missed a small number of trials that may be relevant to our study, but the intent of our analysis was not to be exhaustive; rather, it was to identify whether or not there were issues with registration practices in critical care literature.
While our study describes the frequency of deviation from initially specified sample size and primary outcome, we are unable to determine the reasons behind these changes and cannot exclude the possibility that some alterations—although not explained in the respective manuscripts—were based on sound reasoning. Furthermore, the ability to easily track changes in a trials registry is key to transparency. The ability to track changes is not readily available across all registries, and not in an intuitive fashion. An additional complication is that the terminology permitted (by registries and journals) sometimes results in a lack of clarity relating to key elements (e.g. sample size, primary outcome).
In conclusion, these data suggest that registration of clinical trials in the critically ill is frequently omitted, and among trials that are registered, the timing of registration and the presence of study alterations are usually not apparent in the published paper. There seems little justification for delaying trial registration until after patient enrolment has begun; indeed protocol changes that result in publication of potentially invalid data may be a greater problem than selective reporting, the prevention of which was the main intent of these registries. Changes in trial design occurring after a study has commenced (and certainly after it is complete) should be documented and justified for peer-reviewers and for readers.
References
Sacks H, Chalmers T, Smith H Jr (1982) Randomized versus historical controls for clinical trials. Am J Med 72:233–240
Sozu T, Sugimoto T, Hamasaki T (2010) Sample size determination in clinical trials with multiple co-primary binary endpoints. Stat Med 29(21):2169–2179
De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R et al (2004) Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med 351(12):1250–1251
Abraham E (2005) The AJRCCM in 2005. Am J Respir Crit Care Med 171(1):1–2
Kirillova O (2012) Results and outcome reporting in ClinicalTrials.gov, what makes it happen? PLoS One 7(6):e37847
Mathieu S, Moher D, Altman DG (2009) Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 302(9):977–984
Silverman HJ (2004) The acute respiratory distress syndrome network controversy: lessons and legacy. Curr Opin Crit Care 10:560–564
Silverman WA (2004) Personal reflections on lessons learned from randomized trials involving newborn infants from 1951 to 1967. Clin Trials 1:179–184
Flanagan BM, Philpott S, Strosberg MA (2011) Protecting participants of clinical trials conducted in the intensive care unit. J Intensive Care Med 26(4):237–249
Halpern SD (2002) Prospective preference assessment: a method to enhance the ethics and efficiency of randomized controlled trials. Control Clin Trials 23(3):274–288
Ospina-Tascón GA, Büchele GL, Vincent J-L (2008) Multicenter, randomized, controlled trials evaluating mortality in intensive care: doomed to fail? Crit Care Med 36(4):1311–1322
Hannink G, Gooszen HG, Rovers MM (2013) Comparison of registered and published primary outcomes in randomized clinical trials of surgical interventions. Ann Surg 257(5):818–823
Milette K, Roseman M, Thombs BD (2011) Transparency of outcome reporting and trial registration of randomized controlled trials in top psychosomatic and behavioral health journals: a systematic review. J Psychosom Res 70(3):205–217
Zarin DA, Tse T, Ide NC (2005) Trial registration at ClinicalTrials.gov between May and October 2005. N Engl J Med 353(26):2779–2787
Aberegg SK, Richards DR, O’Brien JM (2010) Delta inflation: a bias in the design of randomized controlled trials in critical care medicine. Crit Care 14(2):R77
Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q et al (2010) Stopping randomized trials early for benefit and estimation of treatment effects. JAMA 303(12):1180–1187
Bassler D, Montori VM, Briel M, Glasziou P, Guyatt G (2008) Early stopping of randomized clinical trials for overt efficacy is problematic. J Clin Epidemiol 61(3):241–246
Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM et al (2009) Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 179(3):220–227
Iwashyna TJ (2010) Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 153:204–205
Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ (2010) Long-term acute care hospital utilization after critical illness. JAMA 303(22):2253–2259
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304(16):1787–1794
Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-granados N, Cooper A et al (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364(14):1293–1304
Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N et al (2012) Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 185(5):517–524
Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ et al (2013) One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation. Ann Intern Med 153(3):167–175
Silverman H (2011) Protecting vulnerable research subjects in critical care trials: enhancing the informed consent process and recommendations for safeguards. Ann Intensive Care 1(1):8
Sekeres M, Gold JL, Chan A-W, Lexchin J, Moher D, Van Laethem MLP et al (2008) Poor reporting of scientific leadership information in clinical trial registers. PLoS One 3(2):e1610
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: This study found that one-third of critical care medicine (CCM) trials published after July 2005 have not been registered; of those that were registered, registration occurred during—or after completion of—patient enrolment in two-thirds of studies. In well over half of trials that were registered, the primary outcome or sample size was potentially altered between initial registration and final publication. These findings suggest serious problems with reporting and conducting randomized controlled trials in CCM.
A related editorial can be found at doi:10.1007/s00134-014-3265-0.
Appendices
Appendix 1: Registration vs non-registration with ICMJE listing status
Registered
ICMJE | |
Anderson (2008) Lancet Neurology | Y |
Arcangeli (2010) Thrombosis Research | N |
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine | Y |
Barbosa (2010) Critical Care | N |
Barraud (2010) Intensive Care Medicine | N |
Beale (2008) Critical Care Medicine | N |
Blaha (2009) Diabetes Care | Y |
Boerma (2010) Critical Care Medicine | N |
Bouadma (2010) Lancet | Y |
Burtin (2009) Critical Care Medicine | N |
Cader (2010) Journal of Physiotherapy | Y |
Cantaluppi (2008) Intensive Care Medicine | N |
Cheung (2010) Critical Care & Resuscitation | N |
Chung (2010) Critical Care Medicine | N |
Cohen (2009) Critical Care (London, England) | N |
Constantin (2010) Critical Care (London, England) | N |
Dongelmans (2009) Anesthesia & Analgesia | Y |
Dubin (2010) Journal of Critical Care | N |
Endre (2010) Kidney International | N |
Figueroa-Casas (2010) Respiratory Care | N |
Frohmader (2010) American Journal of Critical Care | N |
Gerovasili (2009) Critical Care (London, England) | N |
Gupta (2010) Respiratory Care | N |
Hernandez (2010) Chest | Y |
Hochreiter (2009) Critical Care (London, England) | N |
Holzinger (2010) Diabetes Care | Y |
Holzinger (2011) Critical Care Medicine | N |
Investigators (2009) New England Journal of Medicine | Y |
Investigators (2010) JAMA | Y |
Jabre (2009) Lancet | Y |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine | Y |
Jones (2010) JAMA | Y |
Loisa (2007) Critical Care (London, England) | N |
Meisel (2009) American Journal of Respiratory & Critical Care Medicine | Y |
Mentzelopoulos (2009) Archives of Internal Medicine | Y |
Moraes (2009) Jornal de Pediatria | Y |
Morelli (2008) Critical Care (London, England) | N |
Morelli (2009) Critical Care (London, England) | N |
Naidech (2010) Neurocritical Care | N |
Nobre (2008) American Journal of Respiratory & Critical Care Medicine | Y |
Olson (2009) Neurocritical Care | N |
Ouellet (2009) The American Journal of Surgery | Y |
Papazian (2010) New England Journal of Medicine | Y |
Perez-Barcena (2008) Nutrition | N |
Pichamuthu (2010) Clinical Toxicology | Y |
Pieracci (2009) Surgical Infections | N |
Reade (2009) Critical Care (London, England) | N |
Richir (2009) Pharmalogical Research | N |
Robinson (2010) Critical Care (London, England) | N |
Roquilly (2011) JAMA | Y |
Rose (2008) Intensive Care Medicine | N |
Routsi (2010) Critical Care (London, England) | N |
Shariatpanahi (2010) Journal of Critical Care | N |
Staudinger (2010) Critical Care Medicine | N |
Strom (2010) Lancet | Y |
Timsit (2009) JAMA | Y |
van Eijk (2010) Lancet | Y |
Walz (2010) Critical Care Medicine | N |
Xirouchaki (2008) Intensive Care Medicine | N |
Unable to identify registration
ICMJE | |
Acosta-Escribano (2010) Intensive Care Medicine | N |
Baldasso (2009) Intensive Care Medicine | N |
Bellissimo-Rodrigues (2009) Infection Control & Hospital Epidemiology | N |
Bodur (2008) Anaesthesia & Intensive Care | Y |
Boussekey (2008) Intensive Care Medicine | N |
Cabov (2010) Wiener Klinische Wochenschrift | N |
Chanques (2009) Intensive Care Medicine | N |
Chen (2009) Journal of the Chinese Medical Association: JCMA | N |
Chittawatanarat (2010) Asia Pacific Journal of Clinical Nutrition | N |
Cianchi (2010) British Journal of Anaesthesia | Y |
Clec’h (2008) Intensive Care Medicine | N |
Devlin (2010) Critical Care Medicine | N |
Dijkstra (2010) Journal of Clinical Nursing | N |
Fields (2008) Journal of Neuroscience Nursing | N |
Guo (2007) Journal of Huazhong University of Science and Technology Medical Sciences | N |
Henricson (2008) Complementary Therapies in Clinical Practice | N |
Johannigman (2009) Journal of Trauma-Injury Infection & Critical Care | N |
Knowles (2009) Critical Care Medicine | N |
Lucangelo (2008) Critical Care Medicine | N |
Mahdy (2009) Middle East Journal of Anesthesiology | N |
Mao (2010) Chinese Medical Journal | Y |
Montejo (2010) Intensive Care Medicine | N |
Morelli (2008) British Journal of Anaesthesia | Y |
Peng (2010) Cytokine | N |
Peng (2010) International Journal of Artificial Organs | N |
Rowen (2007) Coronary Artery Disease | Y |
Samransamruajkit (2010) Journal of Critical Care | N |
Schroeder (2009) Langenbecks Archives of Surgery | N |
Seder (2010) Critical Care Medicine | N |
Weinberg (2008) Journal of Trauma-Injury Infection & Critical Care | N |
Wiryana (2009) Acta Medica Indonesiana | N |
Appendix 2: Timing of registration
Registered prior to trial enrolment
Anderson (2008) Lancet Neurology |
Barraud (2010) Intensive Care Medicine |
Beale (2008) Critical Care Medicine |
Bouadma (2010) Lancet |
Cheung (2010) Critical Care & Resuscitation |
Endre (2010) Kidney International |
Figueroa-Casas (2010) Respiratory Care |
Investigators (2009) New England Journal of Medicine |
Jabre (2009) Lancet |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine |
Jones (2010) JAMA |
Meisel (2009) American Journal of Respiratory & Critical Care Medicine |
Naidech (2010) Neurocritical Care |
Nobre (2008) American Journal of Respiratory & Critical Care Medicine |
Papazian (2010) New England Journal of Medicine |
Roquilly (2011) JAMA |
Rose (2008) Intensive Care Medicine |
Strom (2010) Lancet |
Timsit (2009) JAMA |
van Eijk (2010) Lancet |
Registered during trial enrolment
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine |
Boerma (2010) Critical Care Medicine |
Chung (2010) Critical Care Medicine |
Dongelmans (2009) Anesthesia & Analgesia |
Dubin (2010) Journal of Critical Care |
Frohmader (2010) American Journal of Critical Care |
Gerovasili (2009) Critical Care (London, England) |
Gupta (2010) Respiratory Care |
Hernandez (2010) Chest |
Holzinger (2010) Diabetes Care |
Holzinger (2011) Critical Care Medicine |
Investigators (2010) JAMA |
Mentzelopoulos (2009) Archives of Internal Medicine |
Morelli (2008) Critical Care (London, England) |
Morelli (2009) Critical Care (London, England) |
Ouellet (2009) The American Journal of Surgery |
Pieracci (2009) Surgical Infections |
Reade (2009) Critical Care (London, England) |
Richir (2009) Pharmalogical Research |
Routsi (2010) Critical Care (London, England) |
Staudinger (2010) Critical Care Medicine |
Walz (2010) Critical Care Medicine |
Xirouchaki (2008) Intensive Care Medicine |
Registered after trial enrolment
Arcangeli (2010) Thrombosis Research |
Barbosa (2010) Critical Care |
Blaha (2009) Diabetes Care |
Burtin (2009) Critical Care Medicine |
Cader (2010) Journal of Physiotherapy |
Cantaluppi (2008) Intensive Care Medicine |
Cohen (2009) Critical Care (London, England) |
Constantin (2010) Critical Care (London, England |
Hochreiter (2009) Critical Care (London, England) |
Loisa (2007) Critical Care (London, England) |
Moraes (2009) Jornal de Pediatria |
Olson (2009) Neurocritical Care |
Perez-Barcena (2008) Nutrition |
Pichamuthu (2010) Clinical Toxicology |
Robinson (2010) Critical Care (London, England) |
Shariatpanahi (2010) Journal of Critical Care |
Appendix 3: Sample size changes
Registered pre-enrolment
Sample size not changed
Anderson (2008) Lancet Neurology |
Investigators (2009) New England Journal of Medicine |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine |
Jones (2010) JAMA |
Papazian (2010) New England Journal of Medicine |
Sample size changed
Barraud 2010 Initial n registered 740 in July 2005. Stopped for futility at 167 patients |
Beale 2008 Registry states 52 patients anticipated (same as published paper), but paper states 344 patients initially estimated to be required |
Cheung 2010 Registry states 180 patients. Changed to 20 patients in published paper due to apparent enrolment challenges |
Endre 2010 Initial n registered 100 patients Feb 2006. Changed to 130 in registry. Final paper reports on 163 patients (no explanation) |
Figueroa-Casas 2010 Initial n registered 224 patients Nov 2006. Final paper reports on 118 patients |
Naidech 2010 No sample size in original registration, but then n = 20 listed in registry in 2010; n = 6 in Sept 2010. Final paper n = 6 |
Nobre 2008 Initial n registered 70 patients Nov 2005. Final paper reports on 79 (>10 % difference so classified as change). Registry changed after study completed |
Roquilly 2011 No sample size in original registration Sept 2006; changed in registry from 180 to 150 in April 2010. Final paper 150 patients |
Rose 2008 Registry anticipated 222 patients. Final paper reports on 102 patients |
Timsit 2009 Initial registry n = 2,000 Dec 200; registry changed to n = 1,600 April 2008. Final paper n = 1,636 |
Van Eijk 2010 Changed in registry from n = 440 (2008) to n = 104 (2010) Final paper n = 109. Stopped early for harm |
Sample size change unclear
Bouadma 2010 No sample size listed in original registration, so considered to be a change. Sample size added to registry on 14 July 2008 |
Jabre 2009 Initial n registered 250 patients Feb 2007. Final paper reports on 655 patients. Unclear if changed; may have projected 400 (200 + 200 in ‘subgroup of interest’), actually studied 465 patients |
Meisel 2009 No sample size in original registration, but then listed in registry May 2011 after completion of enrolment |
Strom 2010 No sample size in original registration April 2007 Final paper n = 140 |
Registered mid-enrolment
Sample size not changed
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine |
Dongelmans (2009) Anesthesia & Analgesia |
Holzinger (2010) Diabetes Care |
Investigators (2010) JAMA |
Mentzelopoulos (2009) Archives of Internal Medicine |
Morelli (2008) Critical Care (London, England) |
Pieracci (2009) Surgical Infections |
Sample size changed
Chung 2010 Initial n registered = 170 in July 2006; enrolment ended May 2009; changed to 62 in Dec 2010; reason in paper = stopped enrolment because increase need rescue in one arm). Possible harm |
Frohmader 2010 Registry 100, paper 45—stopped early for (both) futility and slow recruitment |
Gerovasili 2009 Registry 80 to 52, in Jan 2013. No reason |
Gupta 2010 Registry 100, paper 53; study ran July 2006–Dec 2007; registered Aug 2007. No reason |
Hernandez 2010 78 to 50 in Feb 2009. Reason stopped early for efficacy |
Morelli 2009 Reg nil; reg 30–45 Feb 2008; paper 45 |
Ouellet 2009 Reg nil, then 80 (Mar 2008), then 22 in paper |
Richir 2009 Reg 30, paper 21; could not recruit more |
Routsi 2010 Reg 80 April 2009, stopped recruiting June 2009; 52 Jan 2013; final reg 52; paper 142 did randomize, but lost to follow-up |
Walz 2010 Reg 680; July 2006 850; Jan 2013 960; paper 960 |
Xirouchi 2008 Reg 250; paper 208; enrolment (May 2006–March 2008) needed 100/arm—different to protocol |
Sample size change unclear
Boerma 2012 Unclear: unsure when it appeared in registry; registration (Jun 2007) occurred midway through the study |
Dubin 2010 Unclear: not in original registration; appears in reg. sometime later as 30, and in paper as 25 |
Holzinger 2011 Unclear: (registration—nil; then 66; registration in July 2007 |
Reade 2009 Unclear: nil registered at beginning, appears as 20 in reg and paper |
Staudinger 2010 Unclear: reg—(enrolled from Sep 2005–April 2008; June 2008 150; 150 paper) |
Registered post enrolment
Sample size not changed
Arcangeli (2010) Thrombosis Research |
Barbosa (2010) Critical Care |
Blaha (2009) Diabetes Care |
Burtin (2009) Critical Care Medicine |
Cader (2010) Journal of Physiotherapy |
Cantaluppi (2008) Intensive Care Medicine |
Cohen (2009) Critical Care (London, England) |
Hochreiter (2009) Critical Care (London, England) |
Loisa (2007) Critical Care (London, England) |
Moraes (2009) Jornal de Pediatria |
Pichamuthu (2010) Clinical Toxicology |
Shariatpanahi (2010) Journal of Critical Care |
Sample size changed
Perez-Barcena (2008) Reg 43 on November 2010. Paper 30 |
Robinson (2010) Reg 80 on February 2010. Paper 72. Stated in the paper reason: (we included 80 patients in the trial; eight were transferred before they could participate. The remaining 72 patients were randomized and treated according to the intent-to-treat principle |
Sample size change unclear
Constantin (2010) Unsure when it appeared in registry. Appears as 40 in final registry. Paper 44 |
Olson (2009) Unsure when it appeared in registry. Appears as 67 in final registry. Paper 67 |
Appendix 4: Primary outcome changes
Registered pre-enrolment
Primary outcome not changed
Bouadma (2010) Lancet |
Cheung (2010) Critical Care & Resuscitation |
Figueroa-Casas (2010) Respiratory Care |
Investigators (2009) New England Journal of Medicine |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine |
Jones (2010) JAMA |
Naidech (2010) Neurocritical Care |
Nobre (2008) American Journal of Respiratory & Critical Care Medicine |
Rose (2008) Intensive Care Medicine |
Strom (2010) Lancet |
Timsit (2009) JAMA |
Primary outcome changed
Anderson (2008) Lancet Neurology Initial reg. Sept 2005: Combination death and dependency, according to a 3–6 score on the mRS, at 3 months; paper: proportional change or growth in haematoma volume during the first 24 h after randomization |
Barraud (2010) Intensive Care Medicine Initial reg. July 2005: ICU mortality rate; reg. Sept 2010: ICU mortality rate, time frame 28 days; paper: 28-day mortality |
Beale (2008) Critical Care Medicine Initial reg. Dec 2005: organ dysfunction assessed by daily total SOFA score and by the delta total daily SOFA score. Study hypothesis states 5-day time frame; paper: organ dysfunction evolution assessed by daily total SOFA and delta daily total SOFA score over a study period of maximum 10 days |
Endre (2010) Kidney International Initial reg. Feb 2006: achieved plasma creatinine levels 7 days after randomization; paper: relative average value of creatinine |
Jabre (2009) Lancet Initial reg. Feb 2007. Maximal value of SOFA in the first 48 h of hospitalization; changed July 2008 in reg: maximal value of SOFA at the end of day 2; paper: maximum SOFA score during the first 3 days in the intensive care unit |
Papazian (2010) New England Journal of Medicine Initial reg. Mar 2006: reduction of the mortality rate of ARDS patients at 90 days; paper: death before hospital discharge and within 90 days of enrolment |
van Eijk (2010) Lancet Initial reg. Jun 2008: duration of delirium (time frame end of delirium); reg. April 2009: duration of delirium (time frame 3 months); paper: duration of delirium during hospital admission |
Primary outcome unclear
Meisel (2009) American Journal of Respiratory & Critical Care Medicine Initial reg. Nov 2005: none listed; reg. changed May 2011 to mHLA-DR expression >15,000 molecules per cell at study day 9; paper: mHLA-DR expression. Study ran Nov 2005–Jan 2007 |
Roquilly (2011) JAMA Initial reg. Sep 2006: none listed; reg. changed Nov 2007 to incidence of nosocomial pneumopathy: radiological, clinical, and bacteriological criteria, time frame 28 days; paper: occurrence of hospital-acquired pneumonia within 28 days of randomization. Study ran Nov 2006 to Aug 2009 |
Registered mid-enrolment
Primary outcome not changed
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine |
Boerma (2010) Critical Care Medicine |
Chung (2010) Critical Care Medicine |
Dongelmans (2009) Anesthesia & Analgesia |
Dubin (2010) Journal of Critical Care |
Frohmader (2010) American Journal of Critical Care |
Gerovasili (2009) Critical Care (London, England) |
Gupta (2010) Respiratory Care |
Holzinger (2010) Diabetes Care |
Holzinger (2011) Critical Care Medicine |
Reade (2009) Critical Care (London, England) |
Richir (2009) Pharmalogical Research |
Routsi (2010) Critical Care (London, England) |
Staudinger (2010) Critical Care Medicine |
Walz (2010) Critical Care Medicine |
Primary outcome changed
Investigators (2010) JAMA Initial reg. 2006—hospital mortality; June 2008 hospital mortality capped at 180 days; paper: hospital or 90-day mortality |
Mentzelopoulos (2009) Archives of Internal Medicine Reg. Dec 2006 + survival to discharge or home of facility as well as ROC; reg Dec 2008 + survival to discharge (d/c) or home of facility as well as ROC + adds time of 60 days; paper: ROC and survival to hospital d/c at 60 days |
Morelli (2008) Critical Care (London, England) Reg Mar 2008 hemodynamics 8 h; paper vasoactives |
Morelli (2009) Critical Care (London, England) Reg May 2007 systemic hemodynamics; paper: vasoactives |
Pieracci (2009) Surgical Infections Many primaries in registry (including mortality); a different one—haematocrit in paper |
Xirouchaki (2008) Intensive Care Medicine Registered = PAV success or sedation doses; paper = proportion of patients meeting failure during 48 h |
Primary outcome unclear
Hernandez (2010) Chest Registry Nov 2007 ETT/hosp; Feb 2009 ETT/1 month; paper ETT/unclear |
Ouellet (2009) The American Journal of Surgery Reg: rate of resp distress, Sept 2007, paper mentions goal only |
Registered post-enrolment
Primary outcome not changed
Arcangeli (2010) Thrombosis Research |
Barbosa (2010) Critical Care |
Blaha (2009) Diabetes Care |
Burtin (2009) Critical Care Medicine |
Cader (2010) Journal of Physiotherapy |
Cohen (2009) Critical Care (London, England) |
Constantin (2010) Critical Care (London, England) |
Hochreiter (2009) Critical Care (London, England) |
Loisa (2007) Critical Care (London, England) |
Moraes (2009) Jornal de Pediatria |
Olson (2009) Neurocritical Care |
Perez-Barcena (2008) Nutrition |
Pichamuthu (2010) Clinical Toxicology |
Robinson (2010) Critical Care (London, England) |
Primary outcome changed
Cantaluppi (2008) Intensive Care Medicine The original registry entry (June 2007): reducing need for renal replacement therapy. Paper endpoint: viability of renal cell cultures |
Shariatpanahi (2010) Journal of Critical Care The original registry entry (August 2009): oxygenation, respiratory mechanics, serum inflammatory factors. Paper endpoint: delayed gastric emptying, developing ventilator-associated pneumonia, and clinical outcomes |
Appendix 5: Registration information data
Journal name | Trial # | Registration date | Enrolment start | Enrolment end |
|---|---|---|---|---|
Registered prior to enrolment | ||||
Anderson (2008) Lancet Neurology | NCT00226096 | September 2005 | November 2005 | April 2007 |
Barraud (2010) Intensive Care Medicine | NCT00122408 | July 2005 | February 2006 | March 2008 |
Beale (2008) Critical Care Medicine | ISRCTN27438588 | December 2005 | January 2006 | January 2008 |
Bouadma (2010) Lancet | NCT00472667 | May 2007 | June 2007 | May 2008 |
Cheung (2010) Critical Care & Resuscitation | ACTRN012606000110583 | March 2006 | May 2006 | October 2008 |
Endre (2010) Kidney International | ACTRN012606000058572 | February 2006 | March 2006 | July 2008 |
Figueroa-Casas (2010) Respiratory Care | NCT00400881 | November 2006 | March 2007 | September 2008 |
Investigators (2009) New England Journal of Medicine | NCT00221013 | September 2005 | December 2005 | August 2008 |
Jabre (2009) Lancet | NCT00440102 | February 2007 | April 2007 | February 2008 |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine | NCT00270673 | December 2005 | February 2006 | March 2008 |
Jones (2010) JAMA | NCT00372502 | September 2006 | January 2007 | January 2009 |
Meisel (2009) American Journal of Respiratory & Critical Care Medicine | NCT00252915 | November 2005 | November 2005 | January 2007 |
Naidech (2010) Neurocritical Care | NCT00727090 | July 2008 | August 2008 | February 2009 |
Nobre (2008) American Journal of Respiratory & Critical Care Medicine | NCT00250666 | November 2005 | February 2006 | April 2007 |
Papazian (2010) New England Journal of Medicine | NCT00299650 | March 2006 | March 2006 | March 2008 |
Roquilly (2011) JAMA | NCT00563303 | September 2006 | November 2006 | August 2009 |
Rose (2008) Intensive Care Medicine | ACTRN12605000265673 | August 2005 | January 2006 | December 2006 |
Strom (2010) Lancet | NCT00466492 | April 2007 | April 2007 | December 2008 |
Timsit (2009) JAMA | NCT00417235 | December 2006 | December 2006 | June 2008 |
van Eijk (2010) Lancet | NCT00704301 | June 2008 | November 2008 | January 2010 |
Registered during enrolment | ||||
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine | NCT00248443 | November 2005 | September 2005 | November 2007 |
Boerma (2010) Critical Care Medicine | NCT00493415 | June 2007 | January 2007 | June 2008 |
Chung (2010) Critical Care Medicine | NCT00351741 | July 2006 | April 2006 | May 2009 |
Dongelmans (2009) Anesthesia & Analgesia | ISRCTN65935865 | December 2005 | October 2005 | July 2006 |
Dubin (2010) Journal of Critical Care | NCT00799916 | November 2008 | January 2006 | August 2009 |
Frohmader (2010) American Journal of Critical Care | ACTRN12605000167662 | August 2005 | July 2005 | October 2006 |
Gerovasili (2009) Critical Care (London, England) | NCT00882830 | April 2009 | September 2007 | June 2009 |
Gupta (2010) Respiratory Care | NCT00510991 | August 2007 | July 2006 | December 2007 |
Hernandez (2010) Chest | NCT00557752 | November 2007 | September 2005 | June 2008 |
Holzinger (2010) Diabetes Care | NCT00494078 | June 2007 | June 2006 | August 2008 |
Holzinger (2011) Critical Care Medicine | NCT00500851 | July 2007 | May 2007 | February 2009 |
Investigators (2010) JAMA | NCT00320099 | April 2006 | January 2006 | January 2009 |
Mentzelopoulos (2009) Archives of Internal Medicine | NCT00411879 | December 2006 | June 2006 | March 2007 |
Morelli (2008) Critical Care (London, England) | NCT00639015 | March 2008 | December 2007 | July 2008 |
Morelli (2009) Critical Care (London, England) | NCT00481572 | May 2007 | January 2007 | January 2008 |
Ouellet (2009) The American Journal of Surgery | NCT00530725 | September 2007 | August 2006 | April 2008 |
Pieracci (2009) Surgical Infections | NCT00450177 | March 2007 | January 2006 | December 2007 |
Reade (2009) Critical Care (London, England) | NCT00505804 | July 2007 | April 2006 | August 2008 |
Richir (2009) Pharmalogical Research | NCT00409097 | December 2006 | January 2006 | December 2007 |
Routsi (2010) Critical Care (London, England) | NCT00882830 | April 2009 | September 2007 | June 2009 |
Staudinger (2010) Critical Care Medicine | NCT00529776 | September 2007 | September 2005 | April 2008 |
Walz (2010) Critical Care Medicine | NCT00288418 | February 2006 | December 2005 | July 2007 |
Xirouchaki (2008) Intensive Care Medicine | ISRCTN00104615 | September 2006 | May 2006 | March 2008 |
Registered after enrolment | ||||
Arcangeli (2010) Thrombosis Research | NCT00890214 | April 2009 | September 2007 | May 2008 |
Barbosa (2010) Critical Care | ISRCTN89432944 | November 2009 | March 2007 | December 2007 |
Blaha (2009) Diabetes Care | NCT00764712 | October 2008 | February 2008 | April 2008 |
Burtin (2009) Critical Care Medicine | NCT00695383 | June 2008 | December 2005 | February 2007 |
Cader (2010) Journal of Physiotherapy | NCT00922493 | June 2009 | December 2007 | November 2008 |
Cantaluppi (2008) Intensive Care Medicine | NCT00490477 | June 2007 | January 2006 | April 2007 |
Cohen (2009) Critical Care (London, England) | ISRCTN16080446 | September 2008 | October 2006 | April 2008 |
Constantin (2010) Critical Care (London, England) | NCT01014299 | November 2009 | September 2007 | September 2008 |
Hochreiter (2009) Critical Care (London, England) | ISRCTN10288268 | February 2009 | January 2006 | March 2007 |
Loisa (2007) Critical Care (London, England) | ISRCTN98820688 | September 2006 | July 2005 | April 2006 |
Moraes (2009) Jornal de Pediatria | NCT00549809 | October 2007 | October 2005 | June 2007 |
Olson (2009) Neurocritical Care | NCT00538369 | October 2007 | November 2006 | September 2007 |
Perez-Barcena (2008) Nutrition | NCT01250080 | November 2010 | October 2005 | October 2006 |
Pichamuthu (2010) Clinical Toxicology | ISRCTN84835351 | January 2009 | November 2007 | December 2008 |
Robinson (2010) Critical Care (London, England) | ISRCTN03037804 | February 2010 | February 2006 | March 2009 |
Shariatpanahi (2010) Journal of Critical Care | NCT00958685 | August 2009 | September 2007 | August 2008 |
Appendix 6: Sample size data
Journal name | Published ‘n’ | ‘n’ at time of registration | Any changes in registry (date) | Final ‘n’ recorded in registry (date) |
|---|---|---|---|---|
Registered prior to enrolment | ||||
Anderson (2008) Lancet Neurology | 404 | 400 | No changes | 404 |
Barraud (2010) Intensive Care Medicine | 167 | 740 | No changes | 740 |
Beale (2008) Critical Care Medicine | 50 | 52 | No changes | |
Bouadma (2010) Lancet | 621 | Not listed | No changes | 630 (2008) |
Cheung (2010) Critical Care & Resuscitation | 20 | 180 | No changes | 180 |
Endre (2010) Kidney International | 163 | 100 | 130 | 130 |
Figueroa-Casas (2010) Respiratory Care | 118 | 224 | No changes | |
Investigators (2009) New England Journal of Medicine | 1,508 | 1,500 | No changes | 1508 |
Jabre (2009) Lancet | 655 | 250 | 646 (2007) | 656 (2008) |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine | 348 | 350 | No changes | 350 |
Jones (2010) JAMA | 300 | 300 | No changes | 300 |
Meisel (2009) American Journal of Respiratory & Critical Care Medicine | 38 | None listed | No changes | 38 |
Naidech (2010) Neurocritical Care | 6 | None listed | 20 (2010) | 6 |
Nobre (2008) American Journal of Respiratory & Critical Care Medicine | 79 | 70 | No changes | 70 |
Papazian (2010) New England Journal of Medicine | 340 | 340 | No changes | 340 |
Roquilly (2011) JAMA | 150 | None listed | 180 (2009) | 150 |
Rose (2008) Intensive Care Medicine | 102 | 222 | No changes | |
Strom (2010) Lancet | 140 | None listed | No changes | 140 |
Timsit (2009) JAMA | 1,636 | 2,000 | 1,600 (2008) | 1,600 |
van Eijk (2010) Lancet | 109 | None listed | 440 (2008) | 104 |
Registered during enrolment | ||||
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine | 219 | 206 | No changes | 206 |
Boerma (2010) Critical Care Medicine | 70 | Not Listed | No changes | 70 |
Chung (2010) Critical Care Medicine | 62 | 170 | 62 (2010) | 62 |
Dongelmans (2009) Anesthesia & Analgesia | 128 | 128 | No changes | 128 |
Dubin (2010) Journal of Critical Care | 25 | None listed | No changes | 30 |
Frohmader (2010) American Journal of Critical Care | 45 | 100 | No changes | 100 |
Gerovasili (2009) Critical Care (London, England) | 49 | 80 | No changes | 80 |
Gupta (2010) Respiratory Care | 53 | None listed | No changes | 100 |
Hernandez (2010) Chest | 50 | None listed | 78 (2009) | 50 |
Holzinger (2010) Diabetes Care | 124 | 120 | No changes | 120 |
Holzinger (2011) Critical Care Medicine | 66 | None listed | No changes | 66 |
Investigators (2010) JAMA | 509 | 508 | No changes | 508 |
Mentzelopoulos (2009) Archives of Internal Medicine | 100 | 100 | No changes | 100 |
Morelli (2008) Critical Care (London, England) | 32 | 30 | 32 (2008) | 32 |
Morelli (2009) Critical Care (London, England) | 45 | 30 | 45 (2008) | 45 |
Ouellet (2009) The American Journal of Surgery | 22 | None listed | 80 (2008) | 80 |
Pieracci (2009) Surgical Infections | 200 | 200 | No changes | 200 |
Reade (2009) Critical Care (London, England) | 20 | None listed | No changes | 20 |
Richir (2009) Pharmalogical Research | 21 | 30 | No changes | 30 |
Routsi (2010) Critical Care (London, England) | 142 | 80 | No changes | 80 |
Staudinger (2010) Critical Care Medicine | 150 | None listed | 150 (2008) | 150 |
Walz (2010) Critical Care Medicine | 960 | 680 | 850 (2006) | 850 |
Xirouchaki (2008) Intensive Care Medicine | 208 | 250 | No changes | 208 |
Registered after enrolment | ||||
Arcangeli (2010) Thrombosis Research | 23 | 23 | No changes | 23 |
Barbosa (2010) Critical Care | 23 | 25 | No changes | 25 |
Blaha (2009) Diabetes Care | 120 | 120 | No changes | 120 |
Burtin (2009) Critical Care Medicine | 90 | 90 | No changes | 90 |
Cader (2010) Journal of Physiotherapy | 41 | Not listed | No changes | Not listed |
Cantaluppi (2008) Intensive Care Medicine | 16 | No changes | 16 | |
Cohen (2009) Critical Care (London, England) | 180 | 180 | No changes | 180 |
Constantin (2010) Critical Care (London, England) | 44 | None | No changes | 40 |
Hochreiter (2009) Critical Care (London, England) | 110 | 110 | No changes | 110 |
Loisa (2007) Critical Care (London, England) | 48 | 48 | No changes | 48 |
Moraes (2009) Jornal de Pediatria | 165 | 70 | No changes | 70 |
Olson (2009) Neurocritical Care | 67 | Not listed | No changes | 67 |
Perez-Barcena (2008) Nutrition | 30 | 43 | No changes | 43 |
Pichamuthu (2010) Clinical Toxicology | 60 | 60 | No changes | None listed |
Robinson (2010) Critical Care (London, England) | 72 | 80 | No changes | 80 |
Shariatpanahi (2010) Journal of Critical Care | 32 | 32 | No changes | 32 |
Appendix 7: Primary outcome data
Journal name | Published outcome | Outcome registered at time of registration | Changes identified within the registry (date) | Final registration outcome (date) |
|---|---|---|---|---|
Registered prior to enrolment | ||||
Anderson (2008) Lancet Neurology | The primary efficacy endpoint was the proportional change or growth in haematoma volume during the first 24 h after randomization | Combination death and dependency, according to a 3–5 score on the mRS, at 3 months (July 2007) | Measure: combination death and dependency, according to a 3–6 scores on the modified Rankin score. Time frame 3 months (July 2007) | Measure: combination death and dependency, according to a 3–6 scores on the modified Rankin score. Time frame: 3 months (July 2007) |
Barraud (2010) Intensive Care Medicine | Primary endpoint was 28-day mortality | ICU mortality rate (July 2005) | Intensive care unit (ICU) mortality rate [time frame 28 days] [designated as safety issue: no] (September 2010) | ICU mortality rate [time frame: 28 days] [designated as safety issue: no] (September 2010) |
Beale (2008) Critical Care Medicine | The primary endpoint was organ dysfunction evolution assessed by daily total SOFA and delta daily total SOFA score over a study period of maximum 10 days | Organ dysfunction assessed by daily total SOFA score and by the delta total daily SOFA score (significant reduction). Variables for organ dysfunction (worse parameter per day). (Note: study hypothesis states 5-day time frame) | ||
Bouadma (2010) Lancet | Primary endpoints were death from any cause by days 28 and 60, and number of days without antibiotics at 28 days after inclusion | Exposition to antibiotics, defined by antibiotic-free days [time frame: assessed 28 days after inclusion] Mortality [time frame: at day 28 and day 60] (May 2007) | None noted | Exposition to antibiotics, defined by antibiotic-free days [time frame: assessed 28 days after inclusion] [designated as safety issue: yes] mortality [time frame: at day 28 and day 60] [designated as safety issue: no] (July 2008) |
Cheung (2010) Critical Care & Resuscitation | ICU and hospital LOS and change in composite scores for satisfaction with quality of care of families, intensivists, and bedside nursing staff | Composite score for overall patient/family satisfaction Time point: outcomes are measured at time of patient’s death, or time of patient’s discharge from ICU Primary outcome 2: composite score of staff satisfaction Time point: outcomes are measured at time of patients death, or time of patient’s discharge from ICU Primary outcome 3: length of ICU stay Time point: outcomes are measured at time of patient’s death, or time of patient’s discharge from ICU Primary outcome 4: length of hospital stay Time point: outcomes are measured at time of patient’s death, or time of patient’s discharge from ICU (March 2006) | None noted | Composite score for overall patient/family satisfaction Time point: outcomes are measured at time of patients death, or time of patient’s discharge from ICU Primary outcome 2: composite score of staff satisfaction Time point: outcomes are measured at time of patient’s death, or time of patient’s discharge from ICU Primary outcome 3: length of ICU stay Time point: outcomes are measured at time of patients death, or time of patient’s discharge from ICU Primary outcome 4: length of hospital stay Time point: outcomes are measured at time of patients death, or time of patient’s discharge from ICU (March 2006) |
Endre (2010) Kidney International | The prospectively defined primary outcome was the RAVC | The achieved plasma creatinine levels Time point: 7 days after randomization (Feb 2006) | None listed | Relative average value of plasma creatinine levels Time point: 7 days after randomization |
Figueroa-Casas (2010) Respiratory Care | The primary outcome was duration of weaning (days from first SBT to extubation) | Duration of weaning time Incidence of reintubation | Duration of weaning time [time frame: days] [designated as safety issue: no] Weaning time was determined as number of days from the day of the first SBT to the day of extubation. All patients were followed until extubation | |
Investigators (2009) New England Journal of Medicine | The primary study outcome was death from any cause within 90 days after randomization | Death from all causes at 90 days after randomization (September 2005) | Death from all causes at 90 days after randomization. [Time frame: within 90 days after randomization] [designated as safety issue: no] (February 2009) | Death from all causes at 90 days after randomization. [Time frame, within 90 days after randomization] [designated as safety issue: no] (February 2009) |
Jabre (2009) Lancet | The primary endpoint was the maximum SOFA score during the first 3 days in the ICU | Maximal value of the SOFA in the first 48 h of hospitalization (February 2007) | Maximal value of the SOFA [time frame: at the end of day 2] [designated as safety issue: yes] (July 2008) | Maximal value of SOFA [time frame: at the end of day 2] [designated as safety issue: yes] (July 2008) |
Jansen (2010) American Journal of Respiratory & Critical Care Medicine | In-hospital mortality | In-hospital mortality (December 2005) | In-hospital mortality (January 2007) | In-hospital mortality (January 2007) |
Jones (2010) JAMA | The primary endpoint was absolute in-hospital mortality rate | In-hospital mortality (September 2006) | Mortality [time frame: in-hospital] [designated as safety issue: no] (December 2007) | Mortality [time frame: in-hospital] [designated as safety issue: no] (December 2007) |
Meisel (2009) American Journal of Respiratory & Critical Care Medicine | The primary outcome measure was mHLA-DR expression | None listed | None listed | Reconstitution of monocytic immunity as defined as a mHLA-DR expression greater than 15,000 molecules per cell at study day 9 [time frame: after therapy] [designated as safety issue: no] (May 2011) |
Naidech (2010) Neurocritical Care | For statistical analysis, the primary study outcome was change in serum sodium at 6, 12, 18, 24, 36, and 48 h after enrolment | Change in serum sodium at 6, 12, 18, 24, 26, and 48 h [time frame: 48 h] [designated as safety issue: yes] (July 2008) | Change in serum sodium from baseline to 6 h [time frame: 48 h] [designated as safety issue: yes] (September 2010) | Change in serum sodium from baseline to 6 h [time frame: 48 h] [designated as safety issue: yes] (September 2010) |
Nobre (2008) American Journal of Respiratory & Critical Care Medicine | The primary endpoint was systemic antibiotic exposure, measured using three variables | Exposure to systemic antimicrobial treatment (in duration of antibiotic treatment and total antibiotic exposure) (November 2005) | None listed | Exposure to systemic antimicrobial treatment (in duration of antibiotic treatment and total antibiotic exposure) (November 2005) |
Papazian (2010) New England Journal of Medicine | The primary outcome was the proportion of patients who died before hospital discharge and within 90 days after study enrolment (the 90-day mortality) | Reduction of the mortality rate of ARDS patients at day 90 (March 2006) | Changed October 2008: reduction of the mortality rate of ARDS patients at day 90 [time frame: 36 months] [designated as safety issue: yes] (October 2008) | Changed October 2008: reduction of the mortality rate of ARDS patients at day 90 [time frame: 36 months] [designated as safety issue: yes] (October 2008) |
Roquilly (2011) JAMA | The study’s primary outcome was occurrence of hospital-acquired pneumonia within 28 days of randomization | None listed | Incidence of nosocomial pneumopathy: radiological, clinical, and bacteriological criteria [time frame: 28 days] (November 2007) | Incidence of nosocomial pneumopathy: radiological, clinical, and bacteriological criteria [time frame: 28 days] [designated as safety issue: no] (April 2008) |
Rose (2008) Intensive Care Medicine | The time to separation, defined as time in hours from randomization (immediately following successful completion of the 30-min spontaneous breathing PS test) to the time of declaration of “separation potential” was the primary outcome of interest | Time to readiness for separation (extubation) | Time to readiness for separation (extubation) | |
Strom (2010) Lancet | The primary outcome measure was the number of days without mechanical ventilation (after successful extubation, or removal of ventilator support for patients with tracheostomies) in a 28-day period | Time receiving mechanical ventilation, intensive care and hospital length of stay (April 2007) | Time receiving mechanical ventilation, total intensive care and hospital length of stay [designated as safety issue: no] (March 2010) | Time receiving mechanical ventilation, total intensive care and hospital length of stay [designated as safety issue: no] (March 2010) |
Timsit (2009) JAMA | Major CRIs for comparison of CHGIS vs control dressings | Systemic catheter-related sepsis as defined by a blinded expert panel to unmask differences between chlorhexidine dressings and no chlorhexidine dressings Significant catheter culture ≥103 cfu/ml for noninferiority between 7-day and 3-day catheter-dressing frequencies (December 2006) | Systemic catheter-related sepsis as defined by a blinded expert panel to unmask differences between chlorhexidine dressings and no chlorhexidine dressings [time frame: 48 h] [designated as safety issue: no] Significant catheter culture ≥103 cfu/ml for noninferiority between 7-day and 3-day catheter-dressing frequencies [time frame: 48 h] [designated as safety issue: no] (April 2008) | Systemic catheter-related sepsis as defined by a blinded expert panel to unmask differences between chlorhexidine dressings and no chlorhexidine dressings [time frame: 48 h] [designated as safety issue: no] Significant catheter culture ≥103 cfu/ml for noninferiority between 7-day and 3-day catheter-dressing frequencies [time frame: 48 h] [designated as safety issue: no] (April 2008) |
van Eijk (2010) Lancet | The primary outcome was the duration of delirium during hospital admission (i.e. in the ICU and in the hospital ward combined) | Duration of delirium [time frame: end of delirium] [designated as safety issue: no] (June 2008) | None listed | Duration of delirium [time frame: 3 months] [designated as safety issue: no] (April 2009) |
Registered during enrolment | ||||
Azoulay (2010) American Journal of Respiratory & Critical Care Medicine | The primary endpoint was the intubation rate | Reduction in intubation rate (November 2005) | Reduction in intubation rate [time frame: 28 days] [designated as safety issue: yes] (Feb 2011) | Reduction in intubation rate [time frame: 28 days] [designated as safety issue: yes] (Feb 2011) |
Boerma (2010) Critical Care Medicine | The primary endpoint was MFI within the 24-h study medication period | Increase of MFI by nitroglycerine [time frame: 2 years] (June 2007) | None noted | Increase of MFI by nitroglycerine [time frame: 2 years] [designated as safety issue: no] (May 2008) |
Chung (2010) Critical Care Medicine | The primary endpoint was ventilator-free days in the first 28 days, defined as the number of days after randomization from day 0 to day 28 alive without ventilator assistance for at least 48 consecutive hours | To assess differences in ventilator-free days during the first 28 days between two ventilator strategies (July 2006) | Ventilator-free days during the first 28 days [time frame: 28 days] [designated as safety issue: no] The primary endpoint was ventilator-free days in the first 28 days, defined as the number of days after randomization from day 0 to day 28 alive without ventilator assistance for at least 48 consecutive hours (November 2010) | Ventilator-free days during the first 28 days [time frame: 28 days] [designated as safety issue: no] The primary endpoint was ventilator-free days in the first 28 days, defined as the number of days after randomization from day 0 to day 28 alive without ventilator assistance for at least 48 consecutive hours (November 2010) |
Dongelmans (2009) Anesthesia & Analgesia | The primary endpoint of this study was time until tracheal extubation | Number of ABG analyses Number of audible alarms Number of manual changes in the ventilator settings, including: Switches from PC to PS (only in the control group) Changes in minute ventilation (only in the ASV group) Lowering of PS level (only in the control group) Duration of period of spontaneous mechanical ventilation Duration of total period of tracheal intubation | Number of ABG analyses Number of audible alarms Number of manual changes in the ventilator settings, including: Switches from PC to PS (only in the control group) Changes in minute ventilation (only in the ASV group) Lowering of PS level (only in the control group) Duration of period of spontaneous mechanical ventilation Duration of total period of tracheal intubation | |
Dubin (2010) Journal of Critical Care | Sublingual capillary MFI | Sublingual microcirculation [time frame: 24 h] [designated as safety issue: no] (November 2008) | None listed | Sublingual microcirculation [time frame: 24 h] [designated as safety issue: no] (November 2008) |
Frohmader (2010) American Journal of Critical Care | The primary outcome was frequency of liquid stool (mean number of episodes per patient per day) | A double blind randomized placebo-controlled intervention trial to determine the efficacy of the probiotic VSL #3 in reducing the incidence and/or frequency of diarrhoea in enterally fed critically ill patients | A double blind randomized placebo-controlled intervention trial to determine the efficacy of the probiotic VSL #3 in reducing the incidence and/or frequency of diarrhoea in enterally fed critically ill patients | |
Gerovasili (2009) Critical Care (London, England) | The aim of our study was to assess the effect of EMS on muscle mass preservation in critically ill patients with the use of US | Diagnosis of CIPNM [time frame: June 2009] [designated as safety issue: no] (April 2009) | None listed | Diagnosis of CIPNM [time frame: June 2009] [designated as safety issue: no] (April 2009) |
Gupta (2010) Respiratory Care | Improvement in lung function (FEV1 increased by 50 % compared to admission), ICU stay, hospital stay | Improvement in lung function defined as an increase of at least 50 % in FEV1 as compared to baseline value on admission or an increase in FEV1 to greater than 60 % of predicted value [time frame: time to discharge] Intensive care unit length of stay [time frame: time to discharge] Hospital length of stay [time frame: time to discharge] (August 2007) | None listed | Improvement in lung function defined as an increase of at least 50 % in FEV1 as compared to baseline value on admission or an increase in FEV1 to greater than 60 % of predicted value [time frame: time to discharge] Intensive care unit length of stay [time frame: time to discharge] Hospital length of stay [time frame: time to discharge] (August 2007) |
Hernandez (2010) Chest | The primary endpoint was intubation rate | Intubation rate [time frame: hospital stay] (November 2007) | None listed | Intubation rate [time frame: 1 month] [designated as safety issue: no] (February 2009) |
Holzinger (2010) Diabetes Care | The primary endpoint, percentage of time at 110 mg/dl | Percentage of time of normoglycaemia, defined as glucose levels below 110 mg/dl, during the study period [time frame: 72 h] (June 2007) | Percentage of time of normoglycaemia, defined as glucose levels below 110 mg/dl, during the study period [time frame: 72 h] [designated as safety issue: no] (December 2007) | Percentage of time of normoglycaemia, defined as glucose levels below 110 mg/dl, during the study period [time frame: 72 h] [designated as safety issue: no] (December 2007) |
Holzinger (2011) Critical Care Medicine | The primary outcome was successful implantation of the tube | Success rate of jejunal placement [time frame: 24 h] (July 2007) | None listed | Success rate of jejunal placement [time frame: 24 h] [designated as safety issue: no] (February 2008) |
Investigators (2010) JAMA | The primary outcome measure was in-hospital mortality (or 90-day mortality, whichever occurred first) | In-hospital mortality (April 2006) | In-hospital mortality [time frame: day 180] [designated as safety issue: yes] (June 2008) | In-hospital mortality [time frame: day 180] [designated as safety issue: yes] (June 2008) |
Mentzelopoulos (2009) Archives of Internal Medicine | Primary endpoints were return of spontaneous circulation for 15 min or longer and survival to hospital discharge, defined as presence of an attending physician discharge order to home or to a rehabilitation facility | Survival to discharge either to home or to a rehabilitation facility. Return of spontaneous circulation for at least 15 min (December 2006) | (1) Return of spontaneous circulation for longer than 15 min and (2) survival to discharge either to home or to a rehabilitation facility. [Time frame: 60 days (actual)] [designated as safety issue: no] (July 2008) | (1) Return of spontaneous circulation for longer than 15 min and (2) survival to discharge either to home or to a rehabilitation facility. [Time frame: 60 days (actual)] [designated as safety issue: no] (July 2008) |
Morelli (2008) Critical Care (London, England) | The main endpoint of the present study was the modifications of the PDR and CBI after phenylephrine administration as compared with the norepinephrine group | Systemic and regional hemodynamics [time frame: during the first 12 h from the onset of septic shock] [designated as safety issue: no] (March 2008) | None listed | Systemic and regional hemodynamics [time frame: during the first 12 h from the onset of septic shock] [designated as safety issue: no] (March 2008) |
Morelli (2009) Critical Care (London, England) | The primary endpoint of the present study was the reduction of average open-label NE requirements in patients treated with TP as compared with the AVP or NE group | Systemic and regional hemodynamics [time frame: during the first 48 h from the onset of septic shock] (May 2007) | Systemic and regional hemodynamics [time frame: during the first 48 h from the onset of septic shock] [designated as safety issue: yes] (January 2008) | Systemic and regional hemodynamics [time frame: during the first 48 h from the onset of septic shock] [designated as safety issue: yes] (January 2008) |
Ouellet (2009) The American Journal of Surgery | The primary goals of the OPTICC pilot study were to examine the safety and feasibility of conducting a fully powered noninferiority trial of the treatment of OPTXs in traumatized patients undergoing PPV | Outcome variables: in ventilated patients with small to moderate sized OPTXs, the rate of respiratory distress will not differ between those treated with tube thoracostomy tubes and those not treated but simply observed | Outcome variables: in ventilated patients with small to moderate sized OPTXs, the rate of respiratory distress will not differ between those treated with tube thoracostomy tubes and those not treated but simply observed | |
Pieracci (2009) Surgical Infections | Power analysis was conducted on the basis of the primary outcome of difference in haematocrit, computed at enrolment and weekly thereafter | Weekly haemoglobin, days 0–42 Weekly serum iron concentration, days 0–42 Weekly serum ferritin concentration, days 0–42 Weekly erythrocyte zinc protoporphyrin concentration, days 0–42 Blood transfusion Infection Drug-related constipation Drug-related nausea/vomiting ICU mortality Hospital mortality (March 2007) | None listed | Weekly haemoglobin, days 0–42 Weekly serum iron concentration, days 0–42 Weekly serum ferritin concentration, days 0–42 Weekly erythrocyte zinc protoporphyrin concentration, days 0–42 Blood transfusion Infection Drug-related constipation Drug-related nausea/vomiting ICU mortality Hospital mortality (March 2007) |
Reade (2009) Critical Care (London, England) | The primary endpoint was time from the commencement of study drug to extubation | Time from the commencement of treatment to extubation (July 2007) | Not listed | Time from the commencement of treatment to extubation (July 2007) |
Richir (2009) Pharmalogical Research | Unclear | ADMA concentration | ADMA concentration | |
Routsi (2010) Critical Care (London, England) | The primary endpoint was the diagnosis of CIPNM as assessed with the MRC scale for muscle strength | Diagnosis of CIPNM [time frame: June 2009] [designated as safety issue: no] (April 2009) | Not listed | Diagnosis of CIPNM [time frame: June 2009] [designated as safety issue: no] (April 2009) |
Staudinger (2010) Critical Care Medicine | The primary endpoint was occurrence of VAP | VAP | VAP | |
Walz (2010) Critical Care Medicine | The primary antimicrobial outcome was a dichotomous measure that classified CC as positive (15 cfu) or negative (15 cfu) | Incidence of catheter colonization (February 2006) | None listed | Incidence of catheter colonization (February 2006) |
Xirouchaki (2008) Intensive Care Medicine | The primary endpoint was the proportion of patients meeting failure criteria in each mode during the 48-h study period | PAV success rate Sedation doses | ||
Registered after enrolment | ||||
Arcangeli (2010) Thrombosis Research | Platelet responsiveness | Context and purpose of the study: prospective, randomized comparison of a PGIA versus UFH as circuit anticoagulant. Platelet responsiveness was assessed from peripheral blood by LTA induced by collagen and ADP, at baseline, 4 and 24 h after treatment onset. Platelet function was also assessed in blood samples collected in the circuit before and after the filter | Context and purpose of the study: prospective, randomized comparison of a PGIA versus UFH as circuit anticoagulant. Platelet responsiveness was assessed from peripheral blood by LTA induced by collagen and ADP, at baseline, 4 and 24 h after treatment onset. Platelet function was also assessed in blood samples collected in the circuit before and after the filter | |
Barbosa (2010) Critical Care | The outcomes were plasma phospholipid fatty acid profile, inflammatory mediators in plasma and produced by LPS-stimulated whole blood, routine biochemical and physiological markers, gas exchange, and clinical outcomes | Plasma phospholipid fatty acid status at days 0, 1, 2, and 6 Plasma inflammatory cytokines at days 0, 1, 2, and 6 Inflammatory cytokine production by endotoxin stimulated whole blood at days 0, 1, 2, and 6 Length of ICU and hospital stay (Nov 2009) | None noted | Plasma phospholipid fatty acid status at days 0, 1, 2, and 6 Plasma inflammatory cytokines at days 0, 1, 2, and 6 Inflammatory cytokine production by endotoxin stimulated whole blood at days 0, 1, 2, and 6 Length of ICU and hospital stay (Nov 2009) |
Blaha (2009) Diabetes Care | Effectiveness of different TGC management protocols | The effectiveness of different TGC management protocols [time frame: ICU stay] [designated as safety issue: no] | The effectiveness of different TGC management protocols [time frame: ICU stay] [designated as safety issue: no] | |
Burtin (2009) Critical Care Medicine | The primary outcome was 6MWD as measured at hospital discharge | Six-minute walking distance [time frame: hospital discharge] [designated as safety issue: no] | Six-minute walking distance [time frame: hospital discharge] [designated as safety issue: no] | |
Cader (2010) Journal of Physiotherapy | MIP measured by using a vacuum manometer | Measurement of the MIP (June 2009) | None noted | Measurement of the MIP (June 2009) |
Cantaluppi (2008) Intensive Care Medicine | The primary outcome variable was viability of renal cell cultures exposed to the plasma of septic patients | Verify whether LPS removal with PMX-B hemoperfusion protects from acute renal injury, reducing the need for RRT, in patients with severe sepsis from gram negative infection [time frame: up to 28 days] (21 June 2007) | Number of participants not requiring RRT [time frame: 28 days from the admission] (6 May 2010) | Number of participants not requiring RRT [time frame: 28 days from the admission] (6 May 2010) |
Cohen (2009) Critical Care (London, England) | The primary outcome measure was successful extubation, defined as the ability to maintain spontaneous, unassisted breathing for longer than 48 h after removal of the endotracheal tube | Tolerance of the spontaneous breathing trial: ability to maintain spontaneous breathing for longer than 48 h after extubation (September 2008) | None noted | Tolerance of the spontaneous breathing trial: ability to maintain spontaneous breathing for longer than 48 h after extubation (September 2008) |
Constantin (2010) Critical Care (London, England) | The primary endpoint was the PaO2 value obtained 5 min after tracheal intubation | Oxygenation (PaO2) measured 5 min after the onset of mechanical ventilation [time frame: 5 min after the onset of mechanical ventilation] [designated as safety issue: yes] (November 2009) | None noted | Oxygenation (PaO2) measured 5 min after the onset of mechanical ventilation [time frame: 5 min after the onset of mechanical ventilation] [designated as safety issue: yes] (November 2009) |
Hochreiter (2009) Critical Care (London, England) | Duration of antibiotic therapy | Duration of antibiotic therapy (February 2009) | None listed | Duration of antibiotic therapy. (February 2009) |
Loisa (2007) Critical Care (London, England) | The primary endpoint in the study was the difference in the mean blood glucose levels between the study groups and the occurrence of hyper- and hypoglycaemic episodes | Mean blood glucose levels in study groups and the number of hyperglycaemic (more than 7 mmol/l) and hypoglycaemic (less than 3 mmol/l) episodes (September 2006) | None listed | Mean blood glucose levels in study groups and the number of hyperglycaemic (more than 7 mmol/l) and hypoglycaemic (less than 3 mmol/l) episodes (September 2006) |
Moraes (2009) Jornal de Pediatria | To compare mechanical ventilation support in IMV mode with SIMV + PS in terms of length of time on MV, time taken for weaning, and length of hospital stay | Duration of mechanical ventilation/weaning and length of stay in PICU [time frame: 2 years] (October 2007) | Duration of mechanical ventilation/weaning and length of stay in PICU [time frame: 2 years] [designated as safety issue: yes] (January 2008) | Duration of mechanical ventilation/weaning and length of stay in PICU [time frame: 2 years] [designated as safety issue: yes] (January 2008) |
Olson (2009) Neurocritical Care | The primary endpoint for this study was the total dose of sedative drug (propofol) used in 12 h | How much sedative was infused [time frame: length of stay] | How much sedative was infused [time frame: length of stay] | |
Perez-Barcena (2008) Nutrition | Unclear | Expression of TLR2 and TLR4 in peripheral blood monocytes was determined by flow cytometry | Expression of TLR2 and TLR4 in peripheral blood monocytes was determined by flow cytometry | |
Pichamuthu (2010) Clinical Toxicology | The primary clinical outcome was the incidence of intermediate syndrome | Lower the incidence of intermediate syndrome, measured during hospital stay and determined at discharge Reduce effective circulating organophosphate levels, assayed directly and functionally and measured directly after the infusion of trial or placebo interventions (January 2009) | None listed | Lower the incidence of intermediate syndrome, measured during hospital stay and determined at discharge Reduce effective circulating organophosphate levels, assayed directly and functionally and measured directly after the infusion of trial or placebo interventions (January 2009) |
Robinson (2010) Critical Care (London, England) | The primary endpoint was peak anti-factor Xa levels | Peak anti-factor Xa levels (peak = 4 h post-enoxaparin administration). Levels of anti-factor Xa activity were determined using a validated chromogenic assay kit (COAMATIC Heparin, Chromogenix, Instrumentation Laboratory Company, Lexington, USA) with the substrate S-2732, and the apparatus (STA-R Evolution, Diagnostica Stago, Asnieres, France) (February 2010) | Not listed | Peak anti-factor Xa levels (peak = 4 h post-enoxaparin administration). Levels of anti-factor Xa activity were determined using a validated chromogenic assay kit (COAMATIC Heparin, Chromogenix, Instrumentation Laboratory Company, Lexington, USA) with the substrate S-2732, and the apparatus (STA-R Evolution, Diagnostica Stago, Asnieres, France) (February 2010) |
Shariatpanahi (2010) Journal of Critical Care | Delayed gastric emptying, developing ventilator-associated pneumonia, and clinical outcomes | Changes in oxygenation, respiratory mechanics, and serum inflammatory factors [time frame: 21 days] [designated as safety issue: yes] Oxygenation [time frame: 21 days] [designated as safety issue: yes] | Changes in oxygenation, respiratory mechanics, and serum inflammatory factors [time frame: 21 days] [designated as safety issue: yes] Oxygenation [time frame: 21 days] [designated as safety issue: yes] | |
SOFA Sepsis-related Organ Failure Assessment, LOS length of stay, SBT spontaneous breathing trial, PS pressure support, US ultrasonography, MRC Medical Research Council, VAP ventilator-associated pneumonia, cfu colony-forming units, ABG arterial blood gas, LTA light-transmittance aggregometry, MIP maximal inspiratory pressure, RRT renal replacement therapy, ARDS acute respiratory distress syndrome, LPS lipopolysaccharide
Rights and permissions
About this article
Cite this article
Anand, V., Scales, D.C., Parshuram, C.S. et al. Registration and design alterations of clinical trials in critical care: a cross-sectional observational study. Intensive Care Med 40, 700–722 (2014). https://doi.org/10.1007/s00134-014-3250-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3250-7