Abstract
Purpose
To conduct a multicenter, randomized, placebo-controlled, double-blind, phase II study of BAY41-6551 (NCT01004445), an investigational drug–device combination of amikacin, formulated for inhalation, and a proprietary Pulmonary Drug Delivery System, for the treatment of Gram-negative pneumonia in mechanically ventilated patients.
Methods
Sixty-nine mechanically ventilated patients with Gram-negative pneumonia, a clinical pulmonary infection score ≥6, at risk for multidrug-resistant organisms, were randomized to BAY41-6551 400 mg every 12 h (q12h), 400 mg every 24 h (q24h) with aerosol placebo, or placebo q12h for 7–14 days, plus standard intravenous antibiotics. The combined primary endpoint was a tracheal aspirate amikacin maximum concentration ≥6,400 μg/mL (25 × 256 μg/mL reference minimum inhibitory concentration) and a ratio of area under the aspirate concentration–time curve (0–24 h) to minimum inhibitory concentration ≥100 on day 1.
Results
The primary endpoint was achieved in 50% (6/12) and 16.7% (3/18) of patients in the q12h and q24h groups, respectively. Clinical cure rates, in the 48 patients getting ≥7 days of therapy, were 93.8% (15/16), 75.0% (12/16), and 87.5% (14/16) in the q12h, q24h, and placebo groups, respectively (p = 0.467). By the end of aerosol therapy, the mean number of antibiotics per patient per day was 0.9 in the q12h, 1.3 in the q24h, and 1.9 in the placebo groups, respectively (p = 0.02 for difference between groups). BAY41-6551 was well tolerated and attributed to two adverse events in one patient (mild bronchospasm).
Conclusions
BAY41-6551 400 mg q12h warrants further clinical evaluation.
Similar content being viewed by others
Introduction
Pneumonia originating in healthcare facilities, including hospital-acquired pneumonia, ventilator-associated pneumonia (VAP), and healthcare-associated pneumonia (HCAP), is a serious nosocomial infection, with crude mortality rates in VAP patients of 20–80% [1] and although early reports described an attributable mortality of 27–43%, more recent analyses have suggested a substantially lower percentage of deaths attributable to VAP [2]. Gram-negative pathogens, including multidrug-resistant organisms, account for around 65% of pneumonia cases in the intensive care setting [3].
Aminoglycosides have a suitable antimicrobial spectrum for the treatment of serious Gram-negative infections [4]. However, their use in pneumonia is limited by the risks of nephro-, neuro-, and ototoxicity and by poor penetration into infected lung tissues when administered intravenously [5]. Direct pulmonary administration might be effective by achieving high local concentrations, multiple times above the minimum inhibitory concentration (MIC) of even resistant pathogens. Previous studies of pulmonary antibiotic administration in patients with VAP have been inconclusive and may have been hampered by poor drug delivery, off-label use of nebulized intravenous antibiotics, small sample size, and poorly defined patient populations [6]. However, nebulized antibiotics have demonstrated pharmacokinetic and microbiologic benefits in animal models [7, 8].
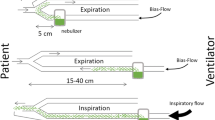
BAY41-6551 (Bayer Schering Pharma/Nektar Therapeutics) is an investigational drug–device combination consisting of amikacin specially formulated for inhalation administered via a proprietary gasless vibrating mesh nebulizer, the Pulmonary Drug Delivery System (PDDS) Clinical device. The PDDS Clinical nebulizer integrates with standard mechanical ventilation equipment (drug administration occurs during inspiratory cycles only), or is fitted to a handheld inhalation unit. In laboratory bench models, both configurations of the PDDS Clinical delivered 50–70% of the nominal dose to the lower airways [9]. The aerosol contains a high proportion of fine particles with a volume median diameter in the range of 3–5 μm, which is optimal for delivery to distal airways [9]. We report a phase II dose-finding study conducted to determine an appropriate BAY41-6551 dosing regimen for phase III development and to define the clinical impact of adjunctive aerosol therapy.
Methods
Study design and treatments
This was a multicenter, randomized, placebo-controlled, double-blind, parallel-group, phase II study at 20 study sites in France, Spain, and the USA (ClinicalTrials.gov registration NCT01004445). Patients were assigned 1:1:1 to BAY41-6551 400 mg every 12 h (q12h regimen), BAY41-6551 400 mg as the first daily dose followed 12 h later with matching aerosol placebo (cycle repeated every 24 h; q24h regimen), or aerosol placebo q12h, for 7–14 days. If the patient was weaned prior to 7 days, an off-ventilator nebulizer configuration was used to complete up to 7 days of inhaled therapy. Each amikacin dose consisted of 3.2 mL of a sulfite-free solution identical in appearance to the aerosol placebo solution. Intravenous antibiotics were administered according to American Thoracic Society/Infectious Disease Society of America guidelines [10]. Of aminoglycosides, only gentamicin or tobramycin could be used. The study was approved by study-site institutional review boards/independent ethics committees; written informed consent was obtained for all patients or their relatives.
Patients
Adults expected to be on mechanical ventilation for ≥3 days were included if they had a clinical diagnosis of Gram-negative hospital-acquired pneumonia (diagnosed ≥48 h after hospital admission), VAP (diagnosed >48 h after intubation), or HCAP (pneumonia in patients with a recent stay in an acute or long-term care facility) [10]; had a clinical pulmonary infection score (CPIS) of ≥6 (as defined by Fartoukh et al. [11] excluding the microbiologic criteria therein); and at least one of four risk factors for Gram-negative multidrug-resistant organisms (hospital stay of at least 5 days or an HCAP diagnosis; antibiotic use in the past 2 weeks; and history of colonization or exposure to multidrug-resistant Gram-negative pathogens) [10]. See supplemental material for exclusion criteria.
Procedures and assessments
Clinical and safety assessments, blood tests, and tracheal aspirate sample cultures (collected according to guidance provided, with or without instilled saline, at the discretion of the investigator) were conducted at baseline, at the end of aerosol treatment (between day 1 and day 14), early post-treatment (3 days after the last aerosol dose), test-of-cure (TOC; 7 ± 1 days after the last dose), and late post-treatment (28–31 days after the first dose). Daily assessments during treatment included serum amikacin trough levels 30 min before the first dose of the day, CPIS calculation, hematology and blood chemistry, urine output, ventilation parameters, chest X-ray, concomitant medications, and adverse events (AEs). Serum creatinine was reviewed before repeat dosing within 12 h of the first daily dose. Daily blood cultures were taken until clear. Tracheal aspirate samples for Gram stain and semiquantitative culture were obtained before the first dose on day 1 and then every other day. Samples on day 1 and day 3 for pharmacokinetic (PK) analysis included tracheal aspirate samples collected at 15 min and 1, 2, 4, 8, and 12 h after the first daily dose; blood collected at 5 min and 1, 2, and 4 h; and all urine collected for 24 h after administration of the first dose of the day. All amikacin concentrations were determined using a validated assay at ICON laboratories (Farmingdale, NY, USA), and tracheal aspirate amikacin concentrations were not corrected for saline dilution.
Analysis populations
The safety population included all randomized patients who received at least one dose of study drug. The PK population comprised all treated patients who had evaluable PK data for any of the three PK specimen types (tracheal aspirate, serum, or urine). The efficacy population was defined as all treated patients who met the enrollment criteria for positive respiratory microbiology and pathogen susceptibility. See supplemental material for analysis populations when samples were missing.
Study endpoints
The primary endpoint was defined as the proportion of patients in the PK-evaluable efficacy population achieving both a tracheal aspirate amikacin maximum concentration (Cmax) of ≥25 times a reference MIC for hospital-acquired organisms of 256 μg/mL (i.e., ≥6,400 μg/mL) and an aspirate area under the concentration–time curve from 0 to 24 h (AUC0–24h) to reference MIC ratio ≥100 on day 1. The targets were based on prior studies of the PK and pharmacodynamic characteristics of aminoglycosides [12–14] and are consistent with the pivotal studies for tobramycin solution for inhalation in cystic fibrosis [14]. We chose a reference MIC of 256 μg/mL because this was the highest amikacin MIC for Pseudomonas aeruginosa and Acinetobacter spp. isolated from North American intensive care units in a separate study [15].
Secondary endpoints included mean amikacin Cmax and AUC0–24h in tracheal aspirates, serum amikacin PK, and cumulative amikacin excretion in 24 h urine collections on day 1 and day 3. At the TOC visit, patients were assessed for clinical cure (complete or partial resolution of signs and symptoms of pneumonia, improvement or lack of progression of all abnormalities on chest X-ray, and no additional intravenous antibiotics since completion of study treatment [16]); microbiologic eradication (confirmed eradication of the original pathogen or presumed eradication in patients with complete or partial resolution of pneumonia); new infections (clinical signs and symptoms of infection with a new pathogen isolated after completing treatment); and superinfection (isolation of a new pathogen during treatment). The total number of systemic antibiotics per patient per day was recorded. An exploratory analysis of CPIS failures was conducted using post hoc scoring developed by the authors: criteria for failure were a rise in CPIS score by ≥2 points on day 3, failure of the CPIS score to drop by ≥1 point on day 5, or failure of the CPIS score to drop by ≥2 points on day 7.
AEs and serious AEs (SAEs) were classified by the investigator as expected or unexpected for the critically ill patient population. Events were considered treatment emergent if they commenced on or after the first dose of study drug and/or up to 3 days after the last dose.
Statistical methods
In October 2005, the study sponsor assessed that the planned sample size of 108 gave inadequate power to demonstrate the therapeutic noninferiority of BAY41-6551. Recruitment was, therefore, stopped early for a descriptive analysis of the PK data. Overall comparisons between treatment arms were performed using a two-sided Fisher’s exact test with a 0.05 significance level. No adjustments for type I errors were made for multiple endpoints. The primary endpoint was analyzed in the efficacy population with evaluable PK using a 90% confidence interval (CI). 90% CIs were also calculated for secondary efficacy endpoints.
Results
Study population and patient characteristics
Patients were enrolled between May 2005 and June 2006 (Fig. 1). Baseline characteristics were generally balanced across treatment groups (Table 1) with the exception of mean weight. P. aeruginosa was the most frequently isolated Gram-negative organism at baseline (24/55 [43.6%] patients), followed by Escherichia coli (14/55 [25.5%]) and Klebsiella spp. (10/55 [18.2%]). A. baumannii, junii, lwoffii, and other Acinetobacter spp. together were isolated in 7/55 (12.7%) of patients. The extent of study drug exposure in the safety population (mean [standard deviation; SD] treatment duration) was 6.6 (1.47), 6.0 (2.11), and 6.5 (2.61) days in the q12h, q24h, and placebo arms, respectively. Four patients in each group transitioned to the handheld configuration because of weaning prior to day 7, and 9 were included in the efficacy population who completed ≥7 days of therapy (Fig. 1).
Patient disposition. a Data for termination from the study used the derived numbers according to the statistical analysis plan. Data for study drug termination were taken directly from the termination sections of the clinical report forms and direct cross-referencing of these data is not possible. The primary reasons for termination from study drug treatment were absence of Gram-negative isolate on initial tracheal aspirate culture (n = 5); AEs/toxicity, including AEs considered unrelated to study medication (n = 3); patient withdrawal (n = 2); investigators’ discretion (n = 1); and other (n = 5). AE adverse event, NA not applicable, PK pharmacokinetic, q12 h every 12 h, q24 h every 24 h. Patients who weaned prior to 7 days, but completed a full course of therapy by transitioning to the handheld configuration are shown in the figure
Pharmacokinetics
The primary endpoint of achieving both tracheal aspirate amikacin concentrations ≥6,400 μg/mL and amikacin aspirate AUC0–24h/256 ≥ 100 at day 1 in the efficacy population with evaluable PK was achieved in 50% (6/12) of the q12h group and in 16.7% (3/18) of the q24h group (90% CI for the difference −61.1 to −5.5; p = 0.102; Table 2). Assessed separately in the efficacy population with evaluable PK, 66.7% of q12h patients and 38.9% of q24h patients had tracheal aspirate amikacin Cmax ≥ 6,400 μg/mL and 75.0 and 33.3%, respectively, had AUC0–24h/256 ≥ 100.
Mean tracheal aspirate amikacin concentrations were higher on day 3 than on day 1, peaked on both days 15–60 min after the end of aerosol administration, and showed a time-dependent decline (Fig. 2 [all treated patients]; Table 2 [PK-evaluable PK population]). Tracheal aspirate concentrations of amikacin did not vary by ventilator mode, with 71% receiving assist-control ventilation and the remainder receiving pressure-support mode. Corresponding serum amikacin Cmax values in the PK-evaluable PK population remained below the recommended maximal trough concentration (10 μg/mL) for systemic amikacin administration (Table 3) [17, 18] and were reached in a median 1–2 h. See supplemental material for urinary pharmacokinetics.
Clinical outcomes
Clinical cure rates at the TOC visit (efficacy population) were 93.8% (15/16), 75.0% (12/16), and 87.5% (14/16) in the q12h, q24h, and placebo groups, respectively (p = 0.467).
In each arm, intravenous antimicrobial use occurred predominantly in the first 7 days and the total number of antimicrobials used per patient from baseline to the TOC visit (efficacy population) was similar in each group: 3.7 (q12h), 4.4 (q24h), and 4.8 (placebo). A post hoc exploratory analysis of variance (ANOVA) (using α = 0.10) showed a gradual but consistent decline in daily per patient intravenous antimicrobial use with amikacin treatment during the aerosol treatment period. The mean number of antibiotics per patient per day for the q12h, q24h, and placebo groups, respectively, was 1.4, 1.5, and 1.6 at day 1 (p = 0.91 for the difference between groups); 1.3, 1.6, and 2.0 at day 3 (p = 0.06); 1.1, 1.4, and 1.7 at day 5 (p = 0.05); and 0.9, 1.3, and 1.9 by the end of aerosol therapy (p = 0.02). We observed that in the first 7 days, antibiotics were added (escalated) in 14, 38, and 58% of the patients in the q12h, q24h, and placebo groups, respectively, while the remainder in each group had antibiotics either stopped or subtracted (de-escalated). Cumulative CPIS failures occurred earlier and in more patients in the placebo compared with the treatment arms, although this did not reach statistical significance (Table 4).
Microbiology
Microbiologic eradication/presumed eradication rates at the TOC visit were 68.8% in both BAY41-6551 groups combined and 62.5% in the placebo group (both study drug groups p > 0.999 vs. placebo). Although initially the microbiologic diagnosis of pneumonia was made by bronchoscopy in about half of all patients, and by tracheal aspirate in the remainder, all cultures to define eradication were performed on either tracheal aspirate or sputum samples. See supplemental material for additional microbiologic data.
Safety and tolerability
BAY41-6551 was relatively well tolerated. In the safety population, 34 patients (50.7%) reported 76 unexpected treatment-emergent AEs. The majority of AEs (80.3%) were mild or moderate in severity. Fifteen severe AEs were reported in six BAY41-6551 400 mg q24h and two placebo patients, of which the most common were septic shock (n = 3) and convulsions (n = 2). Five AEs in four patients were considered possibly or probably related to study drug treatment: three in placebo recipients and two episodes of mild bronchospasm in a patient randomized to BAY41-6551 400 mg q12h.
Twenty-five SAEs were reported in 20 patients; of these, three were possibly treatment related (toxic epidermal necrolysis, acute renal failure, and renal failure in three patients) but occurred in placebo recipients. Three SAEs led to treatment discontinuation: a septic shock event in a patient receiving BAY41-6551 400 mg q24h and renal failure in two placebo recipients.
No group differences were observed in ventilation parameters. Mean serum creatinine levels in all groups remained within the normal range (0.5–1.2 mg/dL). Twelve deaths occurred during the study: three patients in the BAY41-6551 q12h group due to septic shock, throat carcinoma bleeding, and respiratory failure (1 case of each); seven in the BAY41-6551 q24h group due to respiratory arrest, ventricular fibrillation, irreversible secondary subarachnoid hemorrhage, progressive anoxic encephalopathy (one case of each), and three cases of septic shock; and two in the placebo group due to septic shock and toxic epidermal necrolysis (one case each). Only the toxic epidermal necrolysis death in a placebo patient was considered to be possibly treatment related.
Discussion
These results demonstrate that BAY41-6551 400 mg q12h achieves microbiologically relevant amikacin concentrations in the pulmonary secretions of mechanically ventilated patients with Gram-negative pneumonia. Peak serum amikacin concentrations remained several orders of magnitude below those in respiratory secretions and within accepted upper trough concentration limits for systemic amikacin [18], despite the presence of pneumonia, which might enhance drug absorption across the airway surfaces. Peak tracheal aspirate amikacin concentrations were approximately 800 times higher than serum concentrations reported after intravenous amikacin administration in patients with pneumonia, and 4,000 times higher than the concentrations achieved in bronchial secretions after intravenous amikacin administration [19]. These findings are consistent with the literature on inhaled aminoglycosides [20] and suggest limited potential for systemic drug accumulation after multiple BAY41-6551 administrations. The results apply to a study population in which a creatinine level more than 2 mg/dL or urine output less than 0.5 mL/kg/h for 2 consecutive hours was an exclusion criterion; the PK of BAY41-6551 in patients with renal impairment are reported elsewhere [21, 22]. Both BAY41-6551 regimens were well tolerated. The higher number of deaths in the treatment arms compared with the placebo arm was not attributable to the study drug or device and may reflect the limitation of small study populations of critically ill patients inherently at risk of the type of fatalities recorded.
The primary endpoint in this study presupposed that, as with systemic aminoglycosides for Gram-negative infections, optimal treatment outcomes are associated with high ratios of peak concentration to MIC [23]. Moreover, in patients with cystic fibrosis sputum concentrations greater than 25 times the laboratory MIC are needed for reliable eradication of P. aeruginosa [24]. Consequently, we chose a stringent primary PK endpoint, targeting 25 times the MIC of 256 μg/mL [25]. Our finding of clinical success rates that exceeded the percentage of patients achieving our primary PK endpoint indicates that this endpoint may have been too stringent and not predictive of clinical efficacy, particularly because these patients also received systemic antibiotics. Thus we do not believe that a higher dose of inhaled drug is needed. Although we did not demonstrate a statistically significant difference between the two BAY41-6551 regimens on the primary PK outcome, the numerical difference favoring 400 mg q12h (50 vs. 16.7%) suggests it is an appropriate dose for further investigation. For concentration-dependent antibiotics, additional potential advantages of the high respiratory concentrations achieved include reduced likelihood of treatment-emergent resistance and potential activity against pathogens with MICs indicating resistance to systemic therapy on the basis of clinical breakpoints [26].
Although the study was not powered to demonstrate superiority or noninferiority on clinical or microbiologic endpoints, we did observe a potential for clinical benefit of adjunctive aerosol therapy, because of the reduction in systemic antimicrobial use at the end of BAY41-6551 therapy and the lower rate of failure, as defined by CPIS criteria. Collectively, if confirmed, these data suggest a potential role for BAY41-6551 in avoiding both early clinical failure and prolonged use of systemic antibiotic therapy in the treatment of VAP.
Key limitations of this study are the modest sample size, the relatively broad definition of clinical cure at TOC visit, and the allowance of intravenous antibiotic de-escalation, which may have contributed to the lack of differences in clinical cure or microbiologic eradication rates. Amikacin concentrations in suctioned tracheal aspirates probably reflect conditions at the site of infection less accurately than those in epithelial lining fluid samples, which cannot usually be collected serially [27]. Amikacin concentrations in epithelial lining fluid in a different study of BAY41-6551 400 mg q12h in mechanically ventilated patients with Gram-negative pneumonia were lower than those in tracheal aspirates in our study, but still almost four times our 256 μg/mL MIC threshold [28].
In conclusion, adjunctive amikacin delivered via the PDDS Clinical device in mechanically ventilated patients with Gram-negative pneumonia may offer efficacy benefits over systemic antibiotics alone. BAY41-6551 400 mg q12h warrants investigation in larger studies.
References
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Muscadere JG, Day A, Heyland DK (2010) Mortality, attributable mortality and clinical events as endpoints for clinical trials in ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 51:S120–S125
Gaynes R, Edwards JR (2005) Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41:848–854
Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP (2003) Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885–888
Mombelli G, Coppens L, Thys JP, Klastersky J (1981) Anti-Pseudomonas activity in bronchial secretions of patients receiving amikacin or tobramycin as a continuous infusion. Antimicrob Agents Chemother 19:72–75
Dhand R (2007) The role of aerosolized antimicrobials in the treatment of ventilator-associated pneumonia. Respir Care 52:866–884
Ferrari F, Lu Q, Girardi C, Petitjean O, Marquette CH, Wallet F, Rouby JJ, Experimental ICU Study Group (2009) Nebulized ceftazidime in experimental pneumonia caused by partially resistant Pseudomonas aeruginosa. Intensive Care Med 35:1792–1800
Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin MH, Le Naour G, Marquette CH, Rouby JJ (2010) Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 36:1147–1155
Dhand R, Sohal H (2008) Pulmonary Drug Delivery System for inhalation therapy in mechanically ventilated patients. Expert Rev Med Devices 5:9–18
American Thoracic Society, Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Fartoukh M, Maitre B, Honoré S, Cerf C, Zahar JR, Brun-Buisson C (2003) Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med 168:173–179
Thomas JK, Forrest A, Bhavnani SM, Hyatt JM, Cheng A, Ballow CH, Schentag JJ (1998) Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother 42:521–527
Highet VS, Forrest A, Ballow CH, Schentag JJ (1999) Antibiotic dosing issues in lower respiratory tract infection: population-derived area under inhibitory curve is predictive of efficacy. J Antimicrob Chemother 43(Suppl A):55–63
Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW (2002) Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest 122:219–226
Sader H, Jones N (2004) In vitro activity of amikacin tested against bacterial isolates from patients with pneumonia hospitalized in intensive care units. Commissioned report. JMI Laboratories, North Liberty
US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (1998) Guidance for industry: nosocomial pneumonia—developing antimicrobial drugs for treatment. http://www.fda.gov/ohrms/dockets/98fr/2571dft.pdf. Accessed 15 March 2011
Begg EJ, Barclay ML, Kirkpatrick CM (2001) The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 52(Suppl 1):35S–43S
Hammett-Stabler CA, Johns T (1998) Laboratory guidelines for monitoring of antimicrobial drugs. National Academy of Clinical Biochemistry. Clin Chem 44:1129–1140
Santré C, Georges H, Jacquier JM, Leroy O, Beuscart C, Buguin D, Beaucaire G (1995) Amikacin levels in bronchial secretions of 10 pneumonia patients with respiratory support treated once daily versus twice daily. Antimicrob Agents Chemother 39:264–267
Prober CG, Walson PD, Jones J (2000) Technical report: precautions regarding the use of aerosolized antibiotics Committee on Infectious Diseases and Committee on Drugs. Pediatrics 106:E89
Luyt CE, Eldon MA, Stass H, Gribben D, Corkery K, Chastre J (2011) Pharmacokinetics and tolerability of amikacin administered as BAY41-6551 aerosol in mechanically ventilated patients with Gram-negative pneumonia and acute renal failure. J Aerosol Med Pulm Drug Deliv. doi:10.1089/jamp.2010.0860
Stass H, Corkery K, Gribben D, Eldon MA (2011) Pharmacokinetics and tolerability of BAY41-6551 in subjects with chronic kidney disease. J Aerosol Med Pulm Drug Deliv. doi:10.1089/jamp.2010.0859
Moore RD, Lietman PS, Smith CR (1987) Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99
Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, Davis RL (1985) Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis 132:761–765
EUCAST (2009) Aminoglycosides—EUCAST clinical MIC breakpoints 2009-06-15 (v 1.5). http://www.srga.org/eucastwt/MICTAB/MICaminoglycosides.html. Accessed 15 March 2011
Lacy MK, Nicolau DP, Nightingale CH, Quintiliani R (1998) The pharmacodynamics of aminoglycosides. Clin Infect Dis 27:23–27
Kiem S, Schentag JJ (2008) Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36
Luyt CE, Clavel M, Guntupalli K, Johannigman J, Kennedy JI, Wood C, Corkery K, Gribben D, Chastre J (2009) Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Crit Care 13:R200
Acknowledgments
The work of the principal investigators and their study site teams in recruiting patients and conducting the study is gratefully acknowledged. The principal investigators were: A. Anzueto, South Texas Veterans Administration Hospital, San Antonio, TX; K. Guntupalli, Baylor College of Medicine, Houston, TX; R. Hite, Wake Forest University School of Medicine, Winston-Salem, NC; J. Kennedy Jr., VA Medical Center, Birmingham, AL; J. O’Brian, Davis Heart & Lung Research Institute, Columbus, OH; M. Ott, Melbourne Internal Medicine Associates, Melbourne, FL; T. Smith, Albany Medical Center, Albany, NY; J. Carlet, Hôpital Saint Joseph, Paris, France; J. Chastre, Hôpital Pitié Salpétrière, Paris, France; M. Chavel, CHU Dupuytren, Limoges, France; J-Y. Fagon, Hôpital Européen Georges Pompidou, Paris, France; A. Mercat, Centre Hospitalier Universitaire d’Angers, Angers, France; B. Veber, CHU Charles Nicolle, Rouen, France; M. Wolf, Hôpital Bichat-Claude Bernard, Paris, France; M. Daguerre Talou, Hospital Universitario Principe de Asturias, Alcalá de Henares (Madrid), Spain; A. Torres Martí, Hospital Clinic i Provincial, Barcelona, Spain; F. Álvarez Lerma, Hospital del Mar, Barcelona, Spain; A. Martínez Pellús, Hospital Universitario Virgen de la Arrixaca, El Palmar, Spain; J. Cobo Reinoso, Hospital Universitario Ramón y Cajal, Madrid, Spain; and F. Martínez Sagasti, Hospital Clinico San Carlos, Madrid, Spain. The authors would also like to acknowledge Susanna Ryan of Chameleon Communications International Ltd who provided medical writing assistance with funding from Bayer HealthCare. This work was supported by Nektar Therapeutics.
Conflict of interest
This study and the data analysis were sponsored by Nektar Therapeutics. In August 2007, Nektar Therapeutics entered into a commercial agreement with Bayer HealthCare for the joint development of BAY41-6551. A clinical study report was made available to all authors in addition to statistical support for further data queries and the journal has had permission to review the data on request. MN has received consulting fees from Bayer HealthCare Pharmaceuticals Inc. and Nektar Therapeutics, fees from Bayer HealthCare Pharmaceuticals Inc. for the development of education presentations, travel support for meetings, and lectures from Bayer HealthCare Pharmaceuticals Inc., and a research grant from Bayer HealthCare Pharmaceuticals Inc. for the current study. JC has received fees from Bayer HealthCare Pharmaceuticals Inc. and Nektar Therapeutics for lectures, and a research Grant from Nektar Therapeutics. KC was an employee of Nektar Therapeutics during the conduct of the study and is now employed by Novartis Pharmaceuticals Corporation. JBF was an employee of Nektar Therapeutics during the conduct of the study and is now an employee of Aerogen Limited, a stockholder in Nektar Therapeutics and a patent holder for an aerosol delivery system for mechanical ventilation. C-EL and MSG have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niederman, M.S., Chastre, J., Corkery, K. et al. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med 38, 263–271 (2012). https://doi.org/10.1007/s00134-011-2420-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2420-0