Abstract
Purpose
The clinical use of vasoactive drugs is not only intended to improve systemic hemodynamic variables, but ultimately to attenuate derangements in organ perfusion and oxygenation during shock. This review aims (1) to discuss basic physiology with respect to manipulating vascular tone and its effect on the microcirculation, and (2) to provide an overview of available clinical data on the relation between vasoactive drugs and organ perfusion, with specific attention paid to recent developments that have enabled direct in vivo observation of the microcirculation and concepts that have originated from it.
Methods
A MedLine search was conducted for clinical articles in the English language over the last 15 years pertainig to shock, sepsis, organ failure, or critically ill patients in combination with vasoactive drugs and specific variables of organ perfusion/oxygenation (e.g., tonometry, indocyanine clearance, laser Doppler, and sidestream dark field imaging).
Results
Eighty original papers evaluating the specific relationship between organ perfusion/oxygenation and the use of vasoactive drugs were identified and are discussed in light of physiological theory of vasomotor tone.
Conclusions
Solid clinical data in support of the idea that increasing blood pressure in shock improves microcirculatory perfusion/oxygenation seem to be lacking, and such a concept might not be in line with physiological theory of microcirculation as a low-pressure vascular compartment. In septic shock no beneficial effect on microcirculatory perfusion above a mean arterial pressure of 65 mmHg has been reported, but a wide range in inter-individual effect seems to exist. Whether improvement of microcirculatory perfusion is associated with better patient outcome remains to be elucidated.
Similar content being viewed by others
Introduction
Although shock is defined in terms of a critically low blood pressure, a more generally accepted and physiology-based definition of shock is: the inability of the circulation to sustain the cellular respiration needed to maintain normal organ function [1]. This controversy in definition reflects an underlying dilemma with profound effects at bedside. Although intensivists are aware of the physiology-based mechanisms of shock, they have to rely on surrogate global upstream (e.g., blood pressure) and/or downstream (e.g., SvO2) hemodynamic variables to diagnose ‘inadequate’ organ perfusion and guide therapy. Attempts to integrate regional indicators such as intestinal CO2, lactate and indocyanine green (ICG) clearance, which have all been associated with prognosis, have had limited success.
Apart from these general limitations, it becomes clear that in distributive shock shunting in the microcirculation is an intrinsic complexity. Although theoretically the concept of shunting has been well known for many decades [2], new technologies have elucidated the role of ‘microcirculatory weak units.’ In a model of ischemia reperfusion, a speckled pattern of NADH fluorescence and pO2 emerged after re-oxygenation, indicating an inhomogeneous distribution of cells more vulnerable to ischemic insults than others. Microsphere embolization determined these so-called ‘weak units’ at the capillary level [3]. Provided upstream oxygen delivery is adequate, shunted parts of the microcirculation remain hypoxic in the presence of well-maintained venous pO2, explaining the persistence of elevated lactate and regional pCO2 levels [4] not sensed by the downstream variables such as SvO2 [5]. The net systemic result is an inability to increase tissue oxygen uptake, despite efforts to ‘(supra)normalize’ oxygen delivery, but whether this reflects a true extraction deficit as result of a preceded cellular metabolic shutdown or a potentially correctable state of inhomogeneous hypo perfusion remains to be established [6]. Because of the introduction of direct in vivo observation of the microcirculation [7], heterogeneity of blood flow has now become a key feature of distributive shock. It has not only been reported within the microcirculation [8], but also between different organs, within different regions of one organ, in response to therapeutic interventions and in a time-dependent way [4, 9], all challenging the simplicity of systemic hemodynamic monitoring under such conditions.
At first evaluation, these observations seem to add more data to the already dazzling complexity of treatment modalities in the critically ill. However, these new insights have established two main findings: (1) the independent perfusion behavior of the microcirculation in relation to classically measured systemic hemodynamic variables, albeit within certain absolute limits of minimal perfusion pressure [8], and (2) persistence of microcirculatory alterations are associated with morbidity and mortality under various conditions, irrespective of correction of systemic hemodynamics [10, 11]. The issue to be resolved is whether correction of microcirculatory distributive alterations will improve organ function and morbidity. In order to explore this exiting concept, it is not sufficient to ‘simply’ find novel therapeutic approaches that specifically aim to reopen the microcirculation directly. We also have to be prepared to (re)evaluate our current strategies at the bedside in the light of its potential effects from the perspective of the microcirculation and relate it to organ function and outcome.
Administration of vasoactive compounds and fluid therapy are the cornerstone of hemodynamic management of critically ill patients. In this paper we will review reported effects of vasoactive substances on microcirculatory perfusion and tissue oxygenation in shock. These compounds have been advocated to improve systemic perfusion pressure and oxygen delivery, with subsequent improvement of tissue perfusion and oxygen uptake [12]. But do they behave the way we expect them to do? Since shock is a syndrome rather than a well-defined pathophysiologic entity, disease itself as well as treatment might contribute to the clinical picture [13]. An appreciation of basic physiology with respect to manipulating vascular tone and its effect on the microcirculation is followed by a systematic review of the literature. We will focus our review on recent clinical research that includes microcirculatory effects of three classes of vasoactive substances: vasopressors, inotropes and vasodilators, and follow the suggested classification of shock etiology [2]. Preclinical studies will only be discussed in so far as they pertain to the clinical studies.
Materials and methods
In MedLine the following search strategy was performed. Article titles containing shock or sepsis or septic or organ failure or critically ill in combination with vasopressor or vasoactive or inotrope or inotropic or vasodilator or several specific vasoactive drugs [e.g., (nor)adrenaline/(nor)epinephrine, vasopressin, dopamine, dobutamine, nitroglycerin] were selected. The search was restricted to clinical studies in the English language, published over the last 15 years, where indicators of microcirculatory or regional perfusion were specifically mentioned, or in case the effect was evaluated with aid of specific microcirculatory or regional techniques [e.g., tonometry/capnography, ICG, orthogonal polarization spectral (OPS)/side-stream dark field (SDF) imaging, spectrophotometry (Table 1)].
Results
Physiology
Microcirculatory perfusion pressure is the net result of precapillary inflow pressure minus venular outflow pressure. Most of the pressure drop occurs upstream in small arterioles (resistance vessels) as the principal site of active diameter changes to achieve regulation of blood flow. Mean capillary pressure is therefore much closer to venous pressure than to arterial pressure, which is relevant for the maintenance of tissue fluid balance [14]. Under normal conditions sympathic vasoconstriction is reduced in case of venular hypoxia. This signal is forwarded upstream electrophysiologically via endothelial cells connected by gap junctions proximal to arterioles causing upstream dilatation. This process is regulated per microcirculatory unit arising from the respective arteriole feed vessels [15]. It is proposed that the ‘sensor’ for detecting changes in ambient pO2 is located downstream and acts by release of nitric oxide (NO) in the venules [16]. In this respect additional exogenous precapillary and postcapillary vasoconstriction by means of a vasopressive drug results in a reduced net perfusion pressure over the microcirculation [17], despite increment of systemic blood pressure (Fig. 1). At the same time shunting may disrupt the normal venular signaling process for vasodilatation to prevent local hypoxia. Indeed, in ischemia/reperfusion local hypoxia persists in ‘microcirculatory weak units’ after restoration of oxygen delivery in a speckled pattern that appears to be determined at the capillary level, but not at the arteriolar level [3].
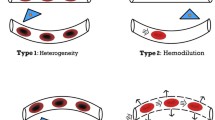
Left panel arteriolar vasodilation increases the opening pressure of the microcirculation as result of a decrease in pressure drop prior to the microvascular compartment. Right panel combined arteriolar and venular increment of vascular tone reduces the net driving pressure over the microvascular compartment (from [17] by permission)
Apart from interference in the complex regulation of tissue perfusion, vasoactive drugs may also influence the homeostasis of tissue oxygenation. Tissue oxygenation is not only dependent on convective properties of red blood cells (flow), but is also determined by diffusion. Given the gas-specific characteristics, oxygen diffusion is related to the pressure gradient and inversely related to the distance between the capillary and the cell (Fig. 2). Closing capillaries in order to maintain perfusion pressure increases diffusion distance and vice versa. Under resting conditions capillaries constantly perform changes in caliber in order to serve both purposes [15].
A third factor that influences microcirculatory oxygen delivery is capillary hematocrit. Mechanical interaction between red blood cells and vessel walls induces the formation of a plasma layer adjacent to the wall and increases hematocrit in the center. Since blood flow-velocity distribution has a parabolic shape, from zero next to the wall to a maximum at the center, the average red cell velocity is higher than overall blood velocity. As a consequence, red cell transit time is reduced. This results in dynamic lowering of the capillary hematocrit in comparison to the entering and discharge hematocrit, and is known as the Fahraeus effect [18]. Additional to this effect, capillary hematocrit is also determined by a phenomenon, originally described by Krogh as ‘plasma skimming’ [19]. At the diverging branches of the capillary network, distribution of red cells is related to the diameter of the daughter vessels (Fig. 3). During in vitro experiments at a systemic hematocrit of 50%, capillary hematocrit ranged from 6.8% during vasoconstriction to 38% under vasodilatation [20], with profound implications on capillary oxygen transport. From the perspective of microcirculation, the dilemma of the use of vasopressors to enhance organ perfusion and oxygenation cannot be better expressed than in the original observations by Krogh: ‘This plasma skimming is usually very pronounced when adrenaline is applied in small drops to muscle arteries of which all branches, even the smallest, react and show a contraction, which in a short time may become complete. The portion of the muscle supplied by the contracting artery becomes blanched and the capillaries often disappear completely from view, while application of adrenaline to capillaries and venules alone shows that these vessels do not react visibly to the substance’ [19].
Under experimental conditions with a systemic hematocrit (HA) of 50%, capillary hematocrit (Hcap) ranges from 6.8% under vasoconstriction to 38% under vasodilation. (From [20] by permission)
Despite abundant experimental data, little is known about human in vivo alterations of vasomotor tone during shock. Direct in vivo observations of the microcirculation in animal endotoxemia demonstrated loss of arterial vasomotion. Interestingly, not all vessels were affected at once, but areas with stagnant blood flow were observed next to areas with brisk flow [21]. In humans, topical endotoxin exposure produced acute venous hyporesponsiveness to NE or sympathic nervous system activation. This was inhibited by glucocorticoids, but not mitigated by NO synthase- or cyclo-oxygenase inhibitors, suggesting other mediators than NO or prostanoids contribute to the overall vascular response to endotoxin [22]. Systemic LPS injection in healthy volunteers blunted the vascular baroreflex response against nitroprusside-induced hypotension and caused complete uncoupling of the cardiac baroreflex from prevailing blood pressure [23]. At present, no direct observations of the microcirculation, other than in tongue or skin, are available from human endotoxemia models.
Human studies
Vasopressors
Norepinephrine (α and β-adrenergic, Table 2) is the most commonly advocated vasopressive drug in septic shock to maintain mean arterial pressure (MAP) at a minimum level of 65 mmHg [12]. Although suggested, improvement of outcome as a result of the use of NE has never been established [24]. Recent studies, which included direct in vivo observation of the microcirculation with Sidestream Dark Field (SDF) imaging, have established that in human sepsis further increment of MAP above 65 mmHg by means of NE does not improve sublingual microcirculatory perfusion, intestinal pCO2 concentrations or arterial lactate levels [25, 26]. This confirmed previous reports in which stepwise increases of MAP with cumulating NE doses did not change arterial lactate levels, skin capillary blood flow, arterial-to-gastric pCO2 gradient (pCO2 g-a), systemic oxygen uptake or renal function [27, 28]. However, substantial inter-individual differences were observed, related to baseline values of microvascular blood flow [25]. Furthermore, these effects may be time and situation dependent [29], and equal changes in MAP may have different drug-related effects on organ perfusion [30]. For renal function an optimal MAP of 75 mmHg in sepsis has been suggested, but direct in vivo observations of the renal microcirculation has not been performed [31]. These studies underline the concept that under conditions of a minimal systemic perfusion pressure, an additional rise in arterial pressure does not automatically improve microcirculatory perfusion in distributive shock, in accordance with physiological theory [17]. Clearly, despite important efforts to compare mortality, systemic and regional perfusion and cardiovascular side effects between (combinations with) NE and other vasopressors, these studies do not elucidate its individual effects on the microcirculation or establish a critical minimum MAP with respect to microcirculatory organ perfusion [32–35]. An observed relation between both hypotension and mortality, as well as vasopressor load and mortality, may be related to the event itself, but may also be a marker for severity of illness [36, 37].
In cardiogenic shock, inotropic agents, but not vasopressors, are advocated [38, 39]. Intra-aortic balloon counter pulsation instantly improved sublingual microcirculatory blood flow in cardiogenic shock, but additional NE dosage was inversely correlated with microcirculatory blood flow [40].
Epinephrine (α and β-adrenergic, Table 2) in the treatment of septic shock has been subject to controversy. Hyperlactatemia during use of epinephrine in sepsis treatment may be caused by tissue hypoxia, but may also be explained by direct metabolic effects [32, 41]. Human data about direct effects of epinephrine on microcirculatory perfusion in sepsis are not available, but only in comparison to NE. In a crossover design, in comparison to NE plus dobutamine, epinephrine was associated with lower intestinal pH (pHi) and higher hepatic venous lactate levels under equal systemic hemodynamic conditions [42], but again interpretation of these data with respect to splanchnic perfusion is complicated by the intrinsic effect of epinephrine to cause hyperlactatemia and systemic acidosis [41, 43].
Phenylephrine (α-adrenergic, Table 2) has been used to increase MAP in human sepsis from 65 to 75 mmHg over a 12-h period [34]. Gastric-to-arterial pCO2 difference, indocyanine clearance, arterial lactate levels and urine output/creatinine clearance were found to be unaltered over time and in comparison to NE, indicating that intestinal microcirculatory organ perfusion neither improved nor deteriorated as a result of a phenylephrine-induced rise in MAP. However, switching from NE to phenylephrine in patients with septic shock while maintaining MAP at 70 mmHg at steady-state systemic hemodynamic variables revealed a rise in pCO2 g-a and a decrease in ICG clearance and urine output/creatinine clearance [44]. These indicators of impaired splanchnic and renal perfusion during phenylephrine infusion disappeared after switch-back to NE again.
In cardiopulmonary bypass (CPB)-induced hypotension, phenylephrine was used to increase MAP from 47 to 68 mmHg [45]. During hypotension, SDF-measured microcirculatory blood flow of sublingual capillaries was unchanged in comparison to pre-CPB hypotension, but after correction with phenylephrine microcirculatory blood flow was significantly reduced. At the same time, global tissue blood flow (laser Doppler) and spectophotometric microvascular hemoglobin oxygen saturation (HbiO2) of the tongue increased, underlining the distributive changes that take place as a result of phenylephrine administration, not sensed in the absence of direct observation of the microcirculation. Interestingly, in an animal model of CPB-induced hypotension, administration of phenylephrine also reduced tissue perfusion to all visceral organs despite correction of MAP from 40 to 65 mmHg [46]. However, correction of hypotension by increasing pump flow improved perfusion of the pancreas, colon and kidneys.
Vasopressin (Table 3) depletion is believed to play a causative role in sepsis-induced vasodilatory shock [47]. In a mixed population with catecholamine-resistant vasodilatory shock, additional low-dose arginine vasopressin (AVP) was associated with an increment of pCO2 g-a over time, but lower than NE alone [48]. Direct in vivo observation of the sublingual microcirculation with OPS imaging in a patient with non-septic distributive shock revealed marked microcirculatory alterations, which did not change after AVP injection [49]. In a randomized controlled open-label trial in patients with septic shock, additional use of AVP disclosed no changes in pCO2 g-a and a higher creatinine clearance in comparison to NE alone [50]. This was in contrast to other reports of patients with septic shock; both additional continuous infusion of low dose AVP as well as replacement of NE by high dose AVP resulted in a significant increase of pCO2 g-a [51, 52]. Equally conflicting data in animal studies are suggested to be related to volume status and cardiac performance [53]. AVP administration in sepsis has been associated with ischemic skin and tongue lesions in up to 30% [54], although in a recent trial the reported incidence was considerably lower and not different from norepinephrine [55].
In post-CPB cardiac surgery patients with high output failure, replacement of NE by AVP during steady-state MAP at 75 mmHg resulted in augmentation of pCO2 g-a and diminished laser Doppler-measured jejunal mucosal perfusion, but this was in conjunction with a significantly lower cardiac index [56]. In hemodynamically stable post-CPB patients, incremental doses of AVP were associated with a decline in renal blood flow and impairment of the renal oxygen demand/supply relationship, in the absence of changes in MAP [57].
Terlipressin (Table 3) not only acts as a long-lasting prodrug for AVP with a high V1a receptor affinity, but is also a fast-acting vasopressor peptide per se that evokes coronary vasoconstriction with reduction in cardiac output [58]. It was originally described as rescue therapy in catecholamine-resistant septic shock [59]. The dilemma of its use in septic shock is outlined in a case report in which a bolus of terlipressin produced significantly higher MAP and urine output while tapering NE doses, but was also associated with a complete shutdown of sublingual microcirculatory perfusion and progressive acidosis [60]. More human data of direct effects of terlipressin on microcirculatory perfusion are not available, but only in comparison to other (combinations of) vasopressors. In septic shock after volume resuscitation, there was no difference between a bolus terlipressin or NE with respect to lactate levels and creatinine clearance [61]. Additional terlipressin to NE in septic shock was accompanied by a rise in bilirubin and aminotransferases [62], but not in pCO2 g-a [63]. As with AVP, ischemic skin lesions are reported frequently [64].
With respect to splanchnic perfusion in relation to terlipressin, it is of note that it has become an established treatment modality in hepatorenal syndrome and gastrointestinal bleeding in liver cirrhosis. This condition is characterized by a marked vasoconstrictive state in the liver in combination with systemic vasoplegia. As opposed to septic shock, its potent ability to reduce splanchnic blood flow has become beneficial under these conditions, either to increase risk of bleeding or to redirect flow to the kidney [65].
Inotropes
Dobutamine (predominantly β2-adrenergic, Table 4) has both inotropic and mild vasodilatory effects. In an open-label setting addition of dobutamine in septic patients was associated with improved OPS-measured sublingual microcirculatory perfusion over time, irrespective of changes in systemic hemodynamic variables [66]. Interestingly, topical application of acetylcholine further improved microcirculatory perfusion, challenging the widespread concept of an irresponsive endothelium in sepsis-induced hypotension. In a crossover setting, dobutamine attenuated intramucosal acidosis during sepsis, whereas dopamine did not change pCO2 g-a under equal effects in systemic hemodynamics [67]. Addition of dobutamine to NE in septic patients revealed a lower pCO2 g-a, but no change in ICG plasma clearance [68, 69]; this could either be due to a direct effect on the microcirculation or a result of an increase in cardiac output. Whether an increase in general splanchnic perfusion indeed is followed by improved organ function is still a matter of debate [70].
Dopamine (β2-adrenergic and dopaminergic, Table 4; in high-dose also α-adrenergic, Table 2) was used in sepsis for many years in low concentrations for its potential beneficial effects on renal function as a result of afferent renal vessel dilatation. However, results of multiple studies have made such an effect of clinical relevance very unlikely [71]. Low-dose dopamine in addition to NE did not alter pHi despite increased overall splanchnic oxygen consumption [72, 73]. High-dose dopamine decreased pHi despite a rise in cardiac output, whereas NE increased pHi at the same MAP [74].
Dopexamine (predominantly dopaminergic, also β2-adrenergic, Table 4) initially was introduced as an inotropic agent with specific pro splanchnic perfusion abilities [75]. However, though dopexamine infusion was associated with a partial correction of spectrophotometric gastric HbiO2, pCO2g-a remained unaltered [76]. This might be compatible with persistence of microcirculatory weak units in combination with shunting, not sensed by spectrophotometry due to incorporation of arteriolar and venular hemoglobin. The same dilemma was illustrated in another study. Dopexamine, in addition to volume loading and dobutamine, increased ICG-derived splanchnic blood flow, but with a concomitant decrease in pHi [77]. Apart from this heterogeneity in blood flow between regional circulation and microcirculation, experimental data also suggested regional heterogeneity in blood flow between the gastric and colon region during the use of dopexamine in sepsis [78].
In postoperative cardiac surgery no difference in pCO2 g-a between low-dose dopexamine and placebo could be detected [79]. In another study pHi did not rise despite an increment in ICG-measured splanchnic blood flow and cardiac output [80]. Apart from thermogenetic effects of dopexamine, this might also be explained by a distributive effect. To date, in postoperative non-cardiac surgery, the use of dopexamine remains a matter of debate [81, 82].
Levosimendan (calcium sensitizer, Table 4) improves cardiac contractility and has slight vasodilatory effects. In patients with sepsis-induced myocardial depression levosimendan in addition to NE plus dobutamine was superior to an incremental dose of dobutamine, with respect to pCO2g-a, laser Doppler-measured gastric mucosal perfusion, arterial lactate levels and creatinine clearance, either as a direct effect or as result of improved cardiac output [83]. After abdominal aortic aneurysm surgery pCO2 g-a was lower in the levosimendan group in comparison to placebo, but despite a higher cardiac output this was not a result of better regional splanchnic perfusion [84]. In an experimental setting attenuation of sepsis-induced cellular hypoxia was observed, but simultaneous SDF-imaging failed to detect changes in microvascular blood flow [85].
Vasodilators
Nitroglycerin (NTG, Table 5) as well as other organic nitrates undergo intracellular metabolism in order to produce nitric oxide (NO)-mediated vasodilatation [86]. Despite the fact that overwhelming NO production is believed to play an important role in sepsis-induced hypotension, it has also been suggested as therapeutic strategy to overcome heterogeneity in microcirculatory blood flow [5, 87]. This dilemma is illustrated by the fact that unselective blocking of NO-synthase indeed was associated with a substantial increase in blood pressure, but also with a higher mortality [88, 89]. The general idea that increasing MAP results in a higher net microcirculatory perfusion pressure is not in line with physiological theory of the microcirculation as a low-pressure vascular compartment [17]. For example, in the brain of healthy volunteers (with intact autoregulation), NE was unable to increase estimated cerebral perfusion pressure despite a rise in MAP, whereas glyceryl trinitrate increased estimated cerebral perfusion pressure as a result of a lower zero flow pressure, even at a lower MAP [90].
In an uncontrolled setting, NTG was administered to septic patients who fulfilled static systemic hemodynamic resuscitation endpoints [91]. Despite fulfilment of these endpoints sublingual microcirculatory flow remained severely impaired. After a single bolus of NTG, instant improvement of microcirculatory blood flow was observed, indicating an NO-mediated endothelial ability for vasodilatation, which based on the analogy of topical acetylcholine challenged the general idea of endothelial hyporesponsiveness in sepsis-induced hypotension [66]. However, in a double-blind placebo-controlled setting, after fulfillment of dynamic systemic hemodynamic resuscitation endpoints, sublingual microcrocirculatory perfusion improved significantly over time, but no effect of NTG in comparison to placebo could be demonstrated [92]. In the uncontrolled setting, baseline values for microvascular blood flow in large vessels was considerably lower in comparison to the controlled setting, suggesting marked differences in systemic volume status.
In cardiogenic shock, impairment of sublingual microcirculation has also been observed and associated with morbidity and mortality [93]. Application of low-dose NTG in addition to standard care for cardiogenic shock was associated with a rise in sublingual perfused capillary density [94]. This was not accompanied by a change in cardiac output or MAP and disappeared after cessation of NTG administration. Interestingly, non-pulsatile systemic circulatory flow support by means of a percutaneous left ventricular assist device after acute myocardial infarction was also reflected in a better sublingual microcirculatory flow score [95].
In the specific setting of gastric tube reconstruction, topical application of NTG improved laser Doppler blood in the fundus without changes in local HbiO2 [96]. However, no effect was observed during intravenous NTG in the same setting, suggesting potential interference with bioactivation [97].
Ketanserin (serotonin 5-HT2 antagonist and weak α1-adrenergic blocker, Table 5) has been used in the treatment of hypertension with a reported beneficial effect on microhemodynamics and hemorheology due vasodilatation and inhibition of platelet aggregation [98]. Administration of ketanserin in hypertensive post-CPB cardiac surgery patients effectively lowered MAP, while sublingual capillary blood flow was preserved [99].
Prostacyclin (cyclic adenosine monophosphate activator, Table 5) was initially propagated as a drug for improvement of oxygen extraction deficits, suspected to cause tissue dysoxia [100]. Since then it has been studied extensively, especially in experimental settings, for its potential beneficial effect on organ perfusion, as it plays a crucial role in the physiological endothelial vasodilatory response to ischemia/reperfusion [101]. Administration of intravenous prostacyclin after conventional resuscitation was associated with higher pHi and indicative for outcome [102]. Similar effects on pHi were reported during aerosolized prostacyclin despite unaltered ICG clearance in patients with pulmonary hypertension [103].
During CPB, infusion of prostacyclin blunted jejunal vasomotion, resulting in a reduced ability to maintain laser Doppler-measured jejunal perfusion constant under variation of blood pressure (autoregulation) [104]. On the other hand, jejunal perfusion increased significantly in parallel to a drop in MAP, but unfortunately pCO2 g-a was not measured to establish the overall effect on jejunal microcirculatory perfusion.
Conclusions
Regardless of practical limitations, there is a growing perception that shock should be defined at the level of the microcirculation. Previous clinical research was limited to surrogate markers of organ microcirculatory blood perfusion such as tonometry, laser Doppler flowmetry and ICG clearance. Recent developments have brought direct in vivo observation of the microcirculation in critically ill patients within reach, albeit limited to accessible organs. To date there are no data available in support of the idea that increasing MAP is beneficial from the perspective of microcirculatory perfusion and/or oxygenation, and such an idea is not in line with the physiological theory of the microcirculation as a low-pressure vascular compartment. In septic shock several reports have demonstrated an absence of effect on microcirculatory perfusion above a MAP of 65 mmHg, but a lower threshold has not been established. The role of vasodilators in sepsis as a therapeutic strategy to recruit ‘microcirculatory weak units’ is yet to be elucidated. In cardiogenic shock neither vasopressors nor inotropic agents have been proven beneficial in terms of organ perfusion, but increasing systemic flow by means of several assist devices are now associated with better microcirculatory flow.
References
Vincent JL, International Sepsis Forum (2001) Hemodynamic support in septic shock. Intensive Care Med 27(Suppl 1):S80–S92
Weil MH, Shubin H (1971) Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol 23:13–23
Ince C, Ashruf JF, Avontuur JA, Wieringa PA, Spaan JA, Bruining HA (1993) Heterogeneity of the hypoxic state in rat heart is determined at capillary level. Am J Physiol 264:H294–H301
Dubin A, Edul VSK, Pozo MO et al (2008) Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med 36:535–542. doi:10.1097/01.CCM.0000300083.74726.43
Buwalda M, Ince C (2002) Opening the microcirculation: can vasodilators be useful in sepsis? Intensive Care Med 28:1208–1217. doi:10.1007/s00134-002-1407-2
Mongardon N, Dyson A, Singer M (2009) Is MOF an outcome parameter or a transient, adaptive state in critical illness? Curr Opin Crit Care 15:431–436. doi:10.1097/MCC.0b013e3283307a3b
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG (1999) Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 5:1209–1212. doi:10.1038/13529
Trzeciak S, Dellinger RP, Parrillo JE et al (2007) Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 49:88–98, 98.e1–2. doi:10.1016/j.annemergmed.2006.08.021
Boerma EC, van der Voort PHJ, Spronk PE, Ince C (2007) Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med 35:1055–1060. doi:10.1097/01.CCM.0000259527.89927.F9
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
Jhanji S, Lee C, Watson D, Hinds C, Pearse RM (2009) Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med 35:671–677. doi:10.1007/s00134-008-1325-z
Dellinger RP, Levy MM, Carlet JM et al (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60. doi:10.1007/s00134-007-0934-2
Ince C (2005) The microcirculation is the motor of sepsis. Crit Care 9(Suppl 4):S13–S19. doi:10.1186/cc3753
Pries AR, Secomb TW, Gaehtgens P (1996) Biophysical aspects of blood flow in the microvasculature. Cardiovasc Res 32:654–667
Segal SS (2005) Regulation of blood flow in the microcirculation. Microcirculation 12:33–45. doi:10.1080/10739680590895028
Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA (1997) Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276:2034–2037
Taylor AE, Moore TM (1999) Capillary fluid exchange. Am J Physiol 277:S203–S210
Fahraeus R (1929) The suspension stability of the blood. Physiol Rev 1929:241–271
Krogh A (1921) Studies on the physiology of capillaries: II. The reactions to local stimuli of the blood-vessels in the skin and web of the frog. J Physiol 55:412–422
Duling BR, Desjardins C (1987) Capillary hematocrit–what does it mean. News Physiol Sci 1987:66–69
Delaunay A, Lebrun J, Delaunay M (1948) Le mode d’action des endotoxines bactériennes IV.Les troubles vase-moteurs chez les animaux intoxiqués et leurs conséquences. J Physiol Paris 40:89–110
Bhagat K, Collier J, Vallance P (1996) Local venous responses to endotoxin in humans. Circulation 94:490–497
Sayk F, Vietheer A, Schaaf B, Wellhoener P, Weitz G, Lehnert H, Dodt C (2008) Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol 295:R891–R898. doi:10.1152/ajpregu.90444.2008
Martin C, Viviand X, Leone M, Thirion X (2000) Effect of norepinephrine on the outcome of septic shock. Crit Care Med 28:2758–2765
Dubin A, Pozo MO, Casabella CA, Palizas F, Murias G, Moseinco MC, Kanoore Edul VS, Estenssoro E, Ince C (2009) Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 13:R92. doi:10.1186/cc7922
Jhanji S, Stirling S, Patel N, Hinds CJ, Pearse RM (2009) The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock*. Crit Care Med 37:1961–1966. doi:10.1097/CCM.0b013e3181a00a1c
LeDoux D, Astiz ME, Carpati CM, Rackow EC (2000) Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 28:2729–2732
Bourgoin A, Leone M, Delmas A, Garnier F, Albanèse J, Martin C (2005) Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med 33:780–786
Albanèse J, Leone M, Garnier F, Bourgoin A, Antonini F, Martin C (2004) Renal effects of norepinephrine in septic and nonseptic patients. Chest 126:534–539. doi:10.1378/chest.126.2.534
Nakajima Y, Baudry N, Duranteau J, Vicaut E (2006) Effects of vasopressin, norepinephrine, and l-arginine on intestinal microcirculation in endotoxemia. Crit Care Med 34:1752–1757. doi:10.1097/01.CCM.0000218812.73741.6C
Deruddre S, Cheisson G, Mazoit JX, Vicaut E, Benhamou D, Duranteau J (2007) Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med 33:1557–1562. doi:10.1007/s00134-007-0665-4
Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J, CAT Study investigators (2008) A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med 34:2226–2234. doi:10.1007/s00134-008-1219-0
Annane D, Vignon P, Renault A et al (2007) Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 370:676–684. doi:10.1016/S0140-6736(07)61344-0
Morelli A, Ertmer C, Rehberg S et al (2008) Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care 12:R143. doi:10.1186/cc7121
De Backer D, Biston P, Devriendt J et al (2010) Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 362:779–789. doi:10.1056/NEJMoa0907118
Dünser MW, Takala J, Ulmer H et al (2009) Arterial blood pressure during early sepsis and outcome. Intensive Care Med 35:1225–1233. doi:10.1007/s00134-009-1427-2
Dünser MW, Ruokonen E, Pettilä V, Ulmer H, Torgersen C, Schmittinger CA, Jakob S, Takala J (2009) Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care 13:R181. doi:10.1186/cc8167
Spahn DR, Cerny V, Coats TJ et al (2007) Management of bleeding following major trauma: a European guideline. Crit Care 11:R17. doi:10.1186/cc5686
Gowda RM, Fox JT, Khan IA (2008) Cardiogenic shock: basics and clinical considerations. Int J Cardiol 123:221–228. doi:10.1016/j.ijcard.2006.03.099
Jung C, Rödiger C, Fritzenwanger M, Schumm J, Lauten A, Figulla HR, Ferrari M (2009) Acute microflow changes after stop and restart of intra-aortic balloon pump in cardiogenic shock. Clin Res Cardiol 98:469–475. doi:10.1007/s00392-009-0018-0
Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE (2005) Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365:871–875. doi:10.1016/S0140-6736(05)71045-X
Meier-Hellmann A, Reinhart K, Bredle DL, Specht M, Spies CD, Hannemann L (1997) Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med 25:399–404
Uusaro A, Takala J (1998) Vasoactive drugs and splanchnic perfusion in septic shock. Crit Care Med 26:1458–1460
Morelli A, Lange M, Ertmer C et al (2008) Short-term effects of phenylephrine on systemic and regional hemodynamics in patients with septic shock: a crossover pilot study. Shock 29:446–451. doi:10.1097/shk.0b013e31815810ff
Maier S, Hasibeder WR, Hengl C, Pajk W, Schwarz B, Margreiter J, Ulmer H, Engl J, Knotzer H (2009) Effects of phenylephrine on the sublingual microcirculation during cardiopulmonary bypass. Br J Anaesth 102:485–491. doi:10.1093/bja/aep018
O’Dwyer C, Woodson LC, Conroy BP, Lin CY, Deyo DJ, Uchida T, Johnston WE (1997) Regional perfusion abnormalities with phenylephrine during normothermic bypass. Ann Thorac Surg 63:728–735
Landry DW, Oliver JA (2001) The pathogenesis of vasodilatory shock. N Engl J Med 345:588–595
Dünser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR (2003) Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation 107:2313–2319. doi:10.1161/01.CIR.0000066692.71008.BB
Dubois MJ, De Backer D, Creteur J, Anane S, Vincent JL (2003) Effect of vasopressin on sublingual microcirculation in a patient with distributive shock. Intensive Care Med 29:1020–1023. doi:10.1007/s00134-003-1742-y
Lauzier F, Lévy B, Lamarre P, Lesur O (2006) Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial. Intensive Care Med 32:1782–1789. doi:10.1007/s00134-006-0378-0
van Haren FMP, Rozendaal FW, van der Hoeven JG (2003) The effect of vasopressin on gastric perfusion in catecholamine-dependent patients in septic shock. Chest 124:2256–2260
Klinzing S, Simon M, Reinhart K, Bredle DL, Meier-Hellmann A (2003) High-dose vasopressin is not superior to norepinephrine in septic shock. Crit Care Med 31:2646–2650. doi:10.1097/01.CCM.0000094260.05266.F4
Asfar P, Bracht H, Radermacher P (2008) Impact of vasopressin analogues on the gut mucosal microcirculation. Best Pract Res Clin Anaesthesiol 22:351–358
Dünser MW, Mayr AJ, Tür A, Pajk W, Barbara F, Knotzer H, Ulmer H, Hasibeder WR (2003) Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med 31:1394–1398. doi:10.1097/01.CCM.0000059722.94182.79
Russell JA, Walley KR, Singer J et al (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887. doi:10.1056/NEJMoa067373
Nygren A, Thorén A, Ricksten SE (2009) Vasopressin decreases intestinal mucosal perfusion: a clinical study on cardiac surgery patients in vasodilatory shock. Acta Anaesthesiol Scand 53:581–588. doi:10.1111/j.1399-6576.2008.01900.x
Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE (2009) Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand 53:1052–1059. doi:10.1111/j.1399-6576.2009.02037.x
Ryckwaert F, Virsolvy A, Fort A, Murat B, Richard S, Guillon G, Colson PH (2009) Terlipressin, a provasopressin drug exhibits direct vasoconstrictor properties: consequences on heart perfusion and performance. Crit Care Med 37:876–881. doi:10.1097/CCM.0b013e31819b8199
O’Brien A, Clapp L, Singer M (2002) Terlipressin for norepinephrine-resistant septic shock. Lancet 359:1209–1210. doi:10.1016/S0140-6736(02)08225-9
Boerma EC, van der Voort PHJ, Ince C (2005) Sublingual microcirculatory flow is impaired by the vasopressin-analogue terlipressin in a patient with catecholamine-resistant septic shock. Acta Anaesthesiol Scand 49:1387–1390. doi:10.1111/j.1399-6576.2005.00752.x
Albanèse J, Leone M, Delmas A, Martin C (2005) Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med 33:1897–1902
Leone M, Albanèse J, Delmas A, Chaabane W, Garnier F, Martin C (2004) Terlipressin in catecholamine-resistant septic shock patients. Shock 22:314–319
Morelli A, Rocco M, Conti G et al (2004) Effects of terlipressin on systemic and regional haemodynamics in catecholamine-treated hyperkinetic septic shock. Intensive Care Med 30:597–604. doi:10.1007/s00134-003-2094-3
Mégarbané H, Barete S, Khosrotehrani K, Izzedine H, Moguelet P, Chosidow O, Frances C, Aractingi S (2009) Two observations raising questions about risk factors of cutaneous necrosis induced by terlipressin (Glypressin). Dermatology 218:334–337. doi:10.1159/000195676
Döhler KD, Meyer M (2008) Vasopressin analogues in the treatment of hepatorenal syndrome and gastrointestinal haemorrhage. Best Pract Res Clin Anaesthesiol 22:335–350
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL (2006) The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34:403–408
Nevière R, Mathieu D, Chagnon JL, Lebleu N, Wattel F (1996) The contrasting effects of dobutamine and dopamine on gastric mucosal perfusion in septic patients. Am J Respir Crit Care Med 154:1684–1688
Joly LM, Monchi M, Cariou A, Chiche JD, Bellenfant F, Brunet F, Dhainaut JF (1999) Effects of dobutamine on gastric mucosal perfusion and hepatic metabolism in patients with septic shock. Am J Respir Crit Care Med 160:1983–1986
Duranteau J, Sitbon P, Teboul JL, Vicaut E, Anguel N, Richard C, Samii K (1999) Effects of epinephrine, norepinephrine, or the combination of norepinephrine and dobutamine on gastric mucosa in septic shock. Crit Care Med 27:893–900
Reinelt H, Radermacher P, Fischer G, Geisser W, Wachter U, Wiedeck H, Georgieff M, Vogt J (1997) Effects of a dobutamine-induced increase in splanchnic blood flow on hepatic metabolic activity in patients with septic shock. Anesthesiology 86:818–824
Debaveye YA, Van den Berghe GH (2004) Is there still a place for dopamine in the modern intensive care unit? Anesth Analg 98:461–468
Meier-Hellmann A, Bredle DL, Specht M, Spies C, Hannemann L, Reinhart K (1997) The effects of low-dose dopamine on splanchnic blood flow and oxygen uptake in patients with septic shock. Intensive Care Med 23:31–37
Olson D, Pohlman A, Hall JB (1996) Administration of low-dose dopamine to nonoliguric patients with sepsis syndrome does not raise intramucosal gastric pH nor improve creatinine clearance. Am J Respir Crit Care Med 154:1664–1670
Marik PE, Mohedin M (1994) The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA 272:1354–1357
Smithies M, Yee TH, Jackson L, Beale R, Bihari D (1994) Protecting the gut and the liver in the critically ill: effects of dopexamine. Crit Care Med 22:789–795
Temmesfeld-Wollbrück B, Szalay A, Mayer K, Olschewski H, Seeger W, Grimminger F (1998) Abnormalities of gastric mucosal oxygenation in septic shock: partial responsiveness to dopexamine. Am J Respir Crit Care Med 157:1586–1592
Seguin P, Laviolle B, Guinet P, Morel I, Mallédant Y, Bellissant E (2006) Dopexamine and norepinephrine versus epinephrine on gastric perfusion in patients with septic shock: a randomized study [NCT00134212]. Crit Care 10:R32. doi:10.1186/cc4827
Tenhunen JJ, Martikainen TJ, Uusaro A, Ruokonen E (2003) Dopexamine reverses colonic but not gastric mucosal perfusion defects in lethal endotoxin shock. Br J Anaesth 91:878–885
Gårdebäck M, Settergren G, Ohquist G, Tirén C (1995) Effect of dopexamine on calculated low gastric intramucosal pH following valve replacement. Acta Anaesthesiol Scand 39:599–604
Uusaro A, Ruokonen E, Takala J (1995) Gastric mucosal pH does not reflect changes in splanchnic blood flow after cardiac surgery. Br J Anaesth 74:149–154
Pearse RM, Belsey JD, Cole JN, Bennett ED (2008) Effect of dopexamine infusion on mortality following major surgery: individual patient data meta-regression analysis of published clinical trials. Crit Care Med 36:1323–1329. doi:10.1097/CCM.0b013e31816a091b
Gopal S, Jayakumar D, Nelson PN (2009) Meta-analysis on the effect of dopexamine on in-hospital mortality. Anaesthesia 64:589–594. doi:10.1111/j.1365-2044.2009.05896.x
Morelli A, De Castro S, Teboul JL et al (2005) Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med 31:638–644. doi:10.1007/s00134-005-2619-z
Leppikangas H, Tenhunen JJ, Lindgren L, Salenius JP, Ruokonen E (2008) Effects of levosimendan on indocyanine green plasma disappearance rate and the gastric mucosal-arterial pCO2 gradient in abdominal aortic aneurysm surgery. Acta Anaesthesiol Scand 52:785–792. doi:10.1111/j.1399-6576.2008.01659.x
Fries M, Ince C, Rossaint R, Bleilevens C, Bickenbach J, Rex S, Mik EG (2008) Levosimendan but not norepinephrine improves microvascular oxygenation during experimental septic shock. Crit Care Med 36:1886–1891. doi:10.1097/CCM.0b013e31817cede9
Münzel T, Wenzel P, Daiber A (2007) Do we still need organic nitrates? J Am Coll Cardiol 49:1296–1298. doi:10.1016/j.jacc.2007.01.007
Trzeciak S, Cinel I, Phillip Dellinger R, Shapiro NI, Arnold RC, Parrillo JE, Hollenberg SM, Microcirculatory Alterations in Resuscitation, Shock (MARS) Investigators (2008) Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med 15:399–413. doi:10.1111/j.1553-2712.2008.00109.x
Bakker J, Grover R, McLuckie A et al (2004) Administration of the nitric oxide synthase inhibitor NG-methyl-l-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002). Crit Care Med 32:1–12. doi:10.1097/01.CCM.0000105118.66983.19
López A, Lorente JA, Steingrub J et al (2004) Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 32:21–30. doi:10.1097/01.CCM.0000105581.01815.C6
Moppett IK, Sherman RW, Wild MJ, Latter JA, Mahajan RP (2008) Effects of norepinephrine and glyceryl trinitrate on cerebral haemodynamics: transcranial Doppler study in healthy volunteers. Br J Anaesth 100:240–244. doi:10.1093/bja/aem374
Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF (2002) Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 360:1395–1396
Boerma EC, Koopmans M, Konijn A et al (2010) Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med 38:93–100. doi:10.1097/CCM.0b013e3181b02fc1
De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL (2004) Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 147:91–99
den Uil CA, Lagrand WK, Spronk PE, van der Ent M, Jewbali LSD, Brugts JJ, Ince C, Simoons ML (2009) Low-dose nitroglycerin improves microcirculation in hospitalized patients with acute heart failure. Eur J Heart Fail 11:386–390. doi:10.1093/eurjhf/hfp021
Lam K, Sjauw KD, Henriques JPS, Ince C, de Mol BA (2009) Improved microcirculation in patients with an acute ST-elevation myocardial infarction treated with the Impella LP2.5 percutaneous left ventricular assist device. Clin Res Cardiol 98:311–318. doi:10.1007/s00392-009-0006-4
Buise MP, Ince C, Tilanus HW, Klein J, Gommers D, van Bommel J (2005) The effect of nitroglycerin on microvascular perfusion and oxygenation during gastric tube reconstruction. Anesth Analg 100:1107–1111. doi:10.1213/01.ANE.0000147665.60613.CA
Buise M, van Bommel J, Jahn A, Tran K, Tilanus H, Gommers D (2006) Intravenous nitroglycerin does not preserve gastric microcirculation during gastric tube reconstruction: a randomized controlled trial. Crit Care 10:R131. doi:10.1186/cc5043
Konishi M, Sakakura M, Tsushima N (1990) Effects of ketanserin on microhemodynamics and hemorheology in patients with essential hypertension. Cardiovasc Drugs Ther 4 Suppl 1:101–104
Elbers PWG, Ozdemir A, van Iterson M, van Dongen EPA, Ince C (2009) Microcirculatory imaging in cardiac anesthesia: ketanserin reduces blood pressure but not perfused capillary density. J Cardiothorac Vasc Anesth 23:95–101. doi:10.1053/j.jvca.2008.09.013
Bihari D, Smithies M, Gimson A, Tinker J (1987) The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N Engl J Med 317:397–403
Scheeren T, Radermacher P (1997) Prostacyclin (PGI2): new aspects of an old substance in the treatment of critically ill patients. Intensive Care Med 23:146–158
Radermacher P, Buhl R, Santak B, Klein M, Kniemeyer HW, Becker H, Tarnow J (1995) The effects of prostacyclin on gastric intramucosal pH in patients with septic shock. Intensive Care Med 21:414–421
Eichelbrönner O, Reinelt H, Wiedeck H, Mezödy M, Rossaint R, Georgieff M, Radermacher P (1996) Aerosolized prostacyclin and inhaled nitric oxide in septic shock–different effects on splanchnic oxygenation? Intensive Care Med 22:880–887
Nygren A, Thorén A, Houltz E, Ricksten SE (2006) Autoregulation of human jejunal mucosal perfusion during cardiopulmonary bypass. Anesth Analg 102:1617–1622. doi:10.1213/01.ANE.0000219596.34753.72
Acknowledgments
We thank M. Koopmans and C. Kesting for their assistance in the design of Fig. 2
Conflict of interest
ECB has no conflict of interest. CI holds a patent on SDF imaging, has stock in Microvision Medical and has received educational grants from Hutchinison Technology, Baxter, Novartis and Eli Lilly.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Boerma, E.C., Ince, C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med 36, 2004–2018 (2010). https://doi.org/10.1007/s00134-010-1970-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1970-x