Abstract
Objective
Acute renal failure can be treated with continuous renal replacement therapy (CRRT) or intermittent haemodialysis (IHD). Whether this choice affects renal recovery has been debated, since it has implications on quality of life and costs. Our objective was to determine the impact of CRRT and IHD on renal recovery.
Design
Nationwide retrospective cohort study between the years 1995 and 2004. Follow-up ranged between 3 months and 10 years.
Setting
Thirty-two Swedish intensive care units.
Patients and participants
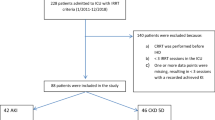
Eligible subjects were adults treated in Swedish general intensive care units with RRT. A total of 2,642 patients from 32 ICUs were included. We then excluded patients with end-stage renal disease (252) and patients lacking a diagnosis in the in-patient register (188). Thus, 2,202 patients were studied. Follow-up was complete.
Interventions
None.
Measurements and results
The primary outcome was renal recovery. Secondarily we studied the mortality of the cohort. There were no differences between IHD and CRRT patients regarding baseline characteristics, such as age, sex and comorbidities. Of the 1,102 patients surviving 90 days after inclusion in the cohort, 944 (85.7%) were treated with CRRT and 158 (14.3%) were treated with IHD. Seventy-eight patients (8.3%; confidence interval, CI, 6.6–10.2), never recovered their renal function in the CRRT group. The proportion was significantly higher among IHD patients, where 26 subjects or 16.5% (CI 11.0–23.2) developed need for chronic dialysis.
Conclusions
The use of CRRT is associated with better renal recovery than IHD, but mortality does not differ between the groups.
Similar content being viewed by others
Introduction
Acute renal failure (ARF) requiring renal replacement therapy (RRT) is common in critically ill patients treated in the intensive care unit (ICU) [1]. The condition often develops as one manifestation of multiple organ failure and is associated with a poor prognosis—the published ICU and hospital mortality rates range from 35% to over 80% [2–4]. The incidence rates for RRT-treated ARF in the general population ranges from 18 to 80 cases/1 million per year in recent studies [3, 5, 6]. There is insufficient knowledge regarding long term outcome of these patients, measured as renal recovery. End-stage renal disease (ESRD) is associated with both costs and impairment of quality of life, making this a paramount issue. The vast discrepancy between reports—some state that one-third of surviving ARF patients had irreversible renal dysfunction and were dependent on dialysis after ICU discharge [7, 8], while others give figures of around 2–8% [2, 3, 9]—raises questions. It should be noted that earlier studies used ICU discharge as a cut-off and only included patients treated with intermittent renal replacement therapy (IHD).
The haemodynamic instability associated with IHD has been found to have an adverse effect on renal recovery [10–12]. This fact, in conjunction with the reports on ICU renal recovery data, led us to hypothesise that the choice of continuous or intermittent RRT affects renal outcome. As CRRT is more expensive than IHD in the ICU, the issue of downstream costs—obviously affected by the need for chronic dialysis—is crucial in order to determine which modality is more economically efficient [13]. The aim of the present study was to assess the impact on renal recovery of (1) the ICU choice of CRRT or IHD and (2) comorbidities. We also studied mortality of the cohort, consisting of Swedish patients with ARF requiring renal replacement therapy in the ICU between 1995 and 2004.
Materials and methods
This study was approved by the Ethics Committee at the Karolinska Institutet, Stockholm, Sweden.
Definition of study cohort
Nationwide data on adult general ICU patients with ARF requiring RRT were put into a database. All adult general ICUs in Sweden were contacted, but some did not have the means to report the required data, whereas other ICUs did have data, but not for the entire period. In total, 32 ICUs provided complete information regarding the subject's unique 10-digit national registration number, admission date to the ICU, date of start of RRT and modality chosen (IHD or CRRT). A total of 2,642 subjects treated between 1995 and 2004 in 32 ICUs were thus included in the study. The date of first recorded ICU admission marked entry into the cohort. The hospitals were classified into three subtypes based upon size and infrastructure according to the National Board of Health and Welfare [14]. Type 1 hospitals are regional/university hospitals, type 2 represents county hospitals and type 3 consists of local hospitals. Regional/university hospitals have larger catchment areas, and are also referral centres for type 2 and 3 hospitals.
The Swedish in-patient register and the population register
Since 1965, the National Board of Health and Welfare has collected data on individual hospital discharges in the in-patient register, described in detail elsewhere [15]. The proportion of the Swedish population covered by this register increased from 60% in 1969 to 85% in 1983, and was 100% by 1987 onwards. The population register is run by Statistics Sweden, which rapidly and continuously records the vital status and addresses of all Swedish citizens and permanent residents.
The Swedish Register for Active Treatment of Uraemia
The Swedish Register for Active Treatment of Uraemia (SRAU) was started in 1991 at a time when there was a lack of reliable data on dialysis and transplantation care in Sweden. The register includes each individual starting active treatment of uraemia with dialysis or kidney transplantation due to chronic renal disease. Every change in treatment (haemodialysis, peritoneal dialysis and transplantation) is reported. Patients are entered into the SRAU only if their need for dialysis is permanent. Date of first treatment marks entry, and discontinuation of treatment leads to removal from the register [16].
Follow-up
The unique 10-digit national registration number assigned to every Swedish citizen ensures identification and follow-up of the patients who have undergone RRT in the ICU [17]. By using the registration number we could link the individual ICU patient data with the in-patient register, the population register and the SRAU. In this study, the in-patient register was used for three reasons: (1) To determine comorbidities recorded during hospital stay present in the cohort, such as diabetes and heart failure. (2) To record the main diagnoses during the ICU stay, i.e. as the patients entered the cohort. (3) Patients that were not found in the in-patient register during the time they were reported to have been in the ICU were excluded from the study. The population register allowed us to record mortality of the cohort. The SRAU provided information on patients with chronic renal disease prior to their ICU stay. These patients were also excluded. The validity of the in-patient registry is high, and it is used for reimbursement, giving the hospitals an economic incentive to be accurate [15, 18].
Furthermore, the SRAU allowed us to find the surviving patients that developed a need for chronic RRT after ARF requiring RRT in the ICU. As only patients with permanent end-stage renal disease are registered in the SRAU, for analysis of renal outcome we included only patients found in the register that remained alive for at least 90 days. A study among all patients who had died with renal disease since 1991 showed that less than 5% of patients starting treatment for chronic renal failure had not been reported to the SRAU [16].
Statistics
The prevalence of permanent renal failure 90 days after initial dialysis was analysed by logistic regression, with covariates categorised as in Table 1. All variables except main diagnosis at ICU were included as covariates. Adjustment for main diagnosis at ICU was accommodated by conditioning. Results are presented as odds ratios with 95% confidence intervals (CI). The risk of late development of renal failure was analysed by Cox regression. Resulting hazard ratios (HR) are presented with 95% CI. Cumulative incidence of permanent renal failure was estimated using a macro provided by Anderson [19]. The effect of dialysis mode in the ICU and subsequent ESRD on mortality was analysed by a Cox regression model, with recruitment to the ESRD group after 90 days as a time-dependent variable. The risk estimates were adjusted for patient characteristics, calendar time and hospital type. Survival curves were estimated through the baseline cumulative hazard function adjusted by Cox regression to the average value for patient characteristics, calendar time and hospital type.
All analyses were performed using the Statistical Analysis System (SAS®) package [20].
Results
In total, 2,642 patients from 32 ICUs were reported to have been treated with RRT between 1995 and 2004. Patients lacking information on main diagnosis (n = 188) in the in-patient register during their reported ICU admission were excluded. Moreover, 252 were dialysis-dependent prior to their treatment in the ICU and were excluded, leaving 2,202 for final analysis (Fig. 1). In only 37 patients was there a switch in treatment from CRRT to IHD, while no patients crossed over from IHD to CRRT.
There were no differences between IHD and CRRT patients regarding baseline characteristics, such as age, sex and comorbidities (Table 1). The main ICU diagnoses were evenly distributed, but sepsis was commonly treated with CRRT and intoxication with IHD. CRRT was more common in type 1 and 2 hospitals. A transition towards CRRT was observed over time (Table 2).
Mortality
A total of 1,102 patients survived and 1,100 patients died within 90 days. The use of IHD or CRRT did not have a significant difference on 90-day mortality (45.7% and 50.6% respectively). Neither did hospital type, where 90-day mortality was 49.7%, 50.6% and 47.8% for type 1, 2 and 3 hospitals respectively. Unsurprisingly, age group, heart failure, but also admittance before the year 2000 correlated with death at 90 days (data not shown). The impact of main ICU diagnosis shows that cancer stands out with the highest 90-day mortality, 72.3% (95% CI 65.5–78.5), whereas intoxication displays the lowest mortality within 90 days, 15.5% (95% CI 8–26).
Among 90-day survivors, long-term mortality (Fig. 2) reveals an elevated risk of death for IHD patients that developed ESRD, with an HR of 2.29 (95% CI 1.27–4.13) compared with CRRT patients with ESRD. In contrast, IHD patients that did not develop ESRD had non-significantly lower mortality than their CRRT counterparts (HR 0.79, 95% CI 0.55–1.14).
Renal outcome
Out of the 1,102 patients surviving 90 days, 944 (85.7%) were treated with CRRT and 158 (14.3%) with IHD. Seventy-eight patients (8.3%, 95% CI 6.6–10.2) never recovered their renal function in the CRRT group. The proportion was significantly higher among IHD patients, where 26 subjects (16.5%, 95% CI 11.0–23.2) developed the need for chronic dialysis. Table 3 highlights the elevated risk for ESRD associated with IHD.
Figure 3 shows “late” development of ESRD among the 998 patients that survived and were not found in the SRAU during the first 90 days. Out of 866 patients on CRRT, 31 (3.6%, 95% CI 2.4–5.0) were later found in the SRAU, compared with 3 out of 132 IHD patients (2.3%, 95% CI 0.5–6.5). Over time, the differing risk for ESRD diminishes between IHD and CRRT groups. The only other variable showing an impact on the need for chronic dialysis was diabetes prior to admission, with an HR of 4.3 (CI 2.1–9.0) after adjusting for age and sex.
Discussion
In this nationwide study we found a significantly higher risk for chronic renal failure with dialysis dependency after critical illness for patients treated with IHD than for patients treated with CRRT. Furthermore, patients treated with IHD that ended up with ESRD showed the highest mortality.
Previous attempts to determine whether the choice of RRT affects outcome have focused on mortality. A recent study showed that if guidelines to improve tolerance and metabolic control were used, 60-day mortality did not differ between CRRT and IHD [21].
A meta-analysis by Kellum et al. compared CRRT with IHD [22]. That investigation found no difference in overall mortality. However, adjusting for study quality and severity of illness, mortality was lower with CRRT (RR 0.72, CI 0.60–0.87, p< 0.01). These findings were criticised by Tonelli and co-workers [23], who, in a meta-analysis of their own, found no survival advantage in unselected critically ill patients with ARF. They did, however, find a non-significantly higher relative risk for ESRD in IHD patients. In the study by Mehta et al., CRRT was found to be beneficial regarding renal recovery. Chronic renal insufficiency at death or hospital discharge was diagnosed in 17% of patients whose therapy was IHD versus only 4% of those whose initial therapy was CRRT (p = 0.01). For patients receiving an adequate trial of monotherapy, recovery of renal function was 92% for CRRT versus 59% for IHD (p< 0.01). Lastly, a higher proportion of subjects crossing over from IHD to CRRT recovered their renal function than patients crossing over in the opposite direction (45 vs. 7%, p< 0.01) [24].
A single-centre study did focus on renal recovery. In 93 patients with ARF it was found that 12.5% of CRRT patients and 64.3% of IHD patients were dialysis dependent at hospital discharge [25]. Although this investigation showed surprisingly high numbers of IHD patients who developed ESRD, it did use hospital discharge, whereas earlier studies of patients treated with IHD [7, 8] used ICU discharge as an end-point and found 30% to be dialysis dependent. In a prospective study of renal recovery Schiffl demonstrated that 33% had mild to moderate failure and 10% had severe renal failure at discharge [26]. However, only one patient progressed to ESRD. In contrast to this, Augustine et al. defined renal recovery as discontinuation of dialysis therapy before discharge from the hospital, and 64% of the survivors were thus considered to have not recovered their renal function [27]. This randomised controlled trial of 80 patients found renal recovery in 9 patients (36% of survivors, or 11% in total) and noted no difference between IHD and CRRT. It is possible that use of both ICU discharge and hospital discharge as cut-off point could lead to an overestimation of the number of subjects needing chronic dialysis. In the present study we define chronic renal failure as a persisting need for dialysis for over 3 months. Linkage to the SRAU enabled us to identify all patients developing the need for chronic dialysis up to 10 years after ICU admission. One could argue that the patients registered in the SRAU later than 90 days after their ICU discharge did not develop kidney failure as a direct result of their ARF requiring treatment in the ICU. For instance, one study followed the patients for 6 months after ICU discharge, but later need for chronic RRT was not considered [3]. However, we believe that long-term renal morbidity must be regarded as a potential result of the acute kidney injury.
The biological hypothesisthat IHD could aggravate the kidney injury in ARF—is based on several observations. The use of IHD in critically ill patients could lead to an increase in oxygen consumption [28] and a reduction in cardiac index in the first hour of IHD [29]. Davenport and co-workers [29] also report reductions in mean arterial pressure, pulmonary capillary wedge pressure and tissue oxygen uptake. IHD is associated with haemodynamic instability during dialysis [30–32]. With CRRT, volume control is continuous, and avoidance of the intravascular volume depletion and hypotension may prevent treatment-associated ischaemic renal injury reported during standard IHD [12].
Renal recovery is an important measure of outcome for many reasons. First, chronic dialysis therapy is associated with significant impairment of health-related quality of life [33–36]. Secondly, it is costly; with annual costs in the range of $51,252–$69,517 [37, 38]. One study showed that the estimated cost per quality-adjusted life-year saved by initiating dialysis was $128,200 [39]. Lastly, the overall mortality of patients with renal failure requiring dialysis exceeds that of the general population. Recent SRAU data report a 27.4% yearly mortality ratio [40].
The limitations of this study needs to be evaluated. We do not have ICU data on these patients, such as RIFLE, APACHE II or SOFA scores, time on ventilator and need for inotropic support. Even though we have type 1, 2 and 3 hospitals, the specific ICU organisation is unknown (availability of intensivists, whether intensivists or nephrologists initiate and execute RRT). Moreover, we lack information regarding dialysis dose and time on dialysis. The risk of residual confounding by time period must also be considered: from 2000 to 2004, 90.7% of all patients were treated with CRRT, compared with 76% in the earlier period. Thus, 48% of the IHD patients were treated during the earlier period, compared with only 23% in the CRRT group. However, the fact that the risk estimates were larger than 2 after adjusting for time period indicates that this cannot be explained solely by residual confounding.
Given the lack of suitable data on severity of illness, the assumption that the patients treated with IHD and CRRT are similar is therefore based on pre-ICU comorbidity information, age, sex and main diagnosis during the ICU stay. Studying the aforementioned data, it does, however, seem likely that the RRT choice was independent of patient status. If anything, the information we do have shows that sicker patients were more often than not treated with CRRT. This is illustrated by the fact that patients on CRRT had more diabetes and heart failure prior to their ICU episode. Also, patients with sepsis and pancreatitis were frequently treated with CRRT. In contrast, the condition with the lowest mortality, intoxication, was often treated with IHD. Some data in this study, including the lack of cross-over, point to the fact that the RRT modality chosen was largely dependent on the type of equipment available at the hospital. Underlying renal disease is probably a predictor of renal recovery. Lack of data regarding this is problematic, but again, nothing in this study indicates that those with underlying disease (acute on chronic) would have been more likely to have been treated with IHD. As mentioned, diabetic patients (with an elevated risk of ESRD) were more often than not treated with CRRT.
A close scrutiny of the difference in renal morbidity between patients treated with IHD or CRRT and the temporal difference in recruitment to chronic renal failure between the two modalities—arouses questions (Fig. 3). Without claiming to have a biological answer to the data in this study, we speculate on the impact of the treatment chosen. Seemingly, IHD has a greater impact on renal function than CRRT which is shown as manifest renal failure developing faster. In contrast, CRRT leads to a smaller imminent need for chronic RRT, but over the years we note an increasing number of patients in the CRRT group ending up with ESRD, and after 10 years the total risk for ESRD is close to that of the IHD group. The assumption that the modality used has a physiologic impact is strengthened by the higher mortality seen in the IHD patients that do develop ESRD.
Conclusions
This large cohort study of intensive care patients with ARF requiring RRT retrospectively studied the outcome in terms of morbidity and mortality. We found that patients ending up in need of long-term dialysis for ESRD more often had been treated with IHD than with CRRT. The higher risk for chronic renal failure with life-long dialysis dependency for patients treated with IHD might lead to a global re-evaluation of which technique should be recommended for critically ill patients.
References
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling CR (2005) Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant 20:354–360
Korkeila M, Ruokonen E, Takala J (2000) Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 26:1824–1831
Vivino G, Antonelli M, Moro ML, Cottini F, Conti G, Bufi M, Cannata F, Gasparetto A (1998) Risk factors for acute renal failure in trauma patients. Intensive Care Med 24:808–814
Feest TG, Round A, Hamad S (1993) Incidence of severe acute renal failure in adults: results of a community based study. BMJ 306:481–483
Liano F, Pascual J (1996) Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50:811–818
Cosentino F, Chaff C, Piedmonte M (1994) Risk factors influencing survival in ICU acute renal failure. Nephrol Dial Transplant 9 Suppl 4:179–182
Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM (1995) Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155:1505–1511
Silvester W, Bellomo R, Cole L (2001) Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med 29:1910–1915
Kelleher SP, Robinette JB, Miller F, Conger JD (1987) Effect of hemorrhagic reduction in blood pressure on recovery from acute renal failure. Kidney Int 31:725–730
Conger JD, Robinette JB, Hammond WS (1991) Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int 39:1087–1097
Manns M, Sigler MH, Teehan BP (1997) Intradialytic renal haemodynamics—potential consequences for the management of the patient with acute renal failure. Nephrol Dial Transplant 12:870–872
Manns B, Doig CJ, Lee H, Dean S, Tonelli M, Johnson D, Donaldson C (2003) Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med 31:449–455
Blomqvist P, Ekbom A, Nyren O, Krusemo UB, Bergstrom R, Adami HO (1999) Survival after rectal cancer: differences between hospital catchment areas. A nationwide study in Sweden. Gut 45:39–44
Nyren O, McLaughlin JK, Gridley G, Ekbom A, Johnell O, Fraumeni JF Jr., Adami HO (1995) Cancer risk after hip replacement with metal implants: a population-based cohort study in Sweden. J Natl Cancer Inst 87:28–33
Schon S, Ekberg H, Wikstrom B, Oden A, Ahlmen J (2004) Renal replacement therapy in Sweden. Scand J Urol Nephrol 38:332–339
Lunde AS, Lundeborg S, Lettenstrom GS, Thygesen L, Huebner J (1980) The person-number systems of Sweden, Norway, Denmark, and Israel. Vital Health Stat 2 2:1–59
Serden L, Lindqvist R, Rosen M (2005) [Benefits with well-educated medical secretaries. Improved coding in the patient registry following a course in classification and care documentation]. Lakartidningen 102:1530, 1533–1534, 1536–1537
Anderson WN (2000) Algorithms for actuarial and actual analysis. Proceedings of 8th Annual Western Users of SAS Software (WUSS):128–133
SAS/STAT® version 9.1. SAS Institute Inc, Cary, NC
Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF (2006) Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 368:379–385
Kellum JA, Angus DC, Johnson JP, Leblanc M, Griffin M, Ramakrishnan N, Linde-Zwirble WT (2002) Continuous versus intermittent renal replacement therapy: a meta-analysis. Intensive Care Med 28:29–37
Tonelli M, Manns B, Feller-Kopman D (2002) Acute renal failure in the intensive care unit: a systematic review of the impact of dialytic modality on mortality and renal recovery. Am J Kidney Dis 40:875–885
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, Kaplan RM (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60:1154–1163
Jacka MJ, Ivancinova X, Gibney RT (2005) Continuous renal replacement therapy improves renal recovery from acute renal failure. Can J Anaesth 52:327–332
Schiffl H (2006) Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant 21:1248–1252
Augustine JJ, Sandy D, Seifert TH, Paganini EP (2004) A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 44:1000–1007
Van der Schueren G, Diltoer M, Laureys M, Huyghens L (1996) Intermittent hemodialysis in critically ill patients with multiple organ dysfunction syndrome is associated with intestinal intramucosal acidosis. Intensive Care Med 22:747–751
Davenport A, Will EJ, Davidson AM (1993) Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med 21:328–338
Lameire N, Van Biesen W, Vanholder R, Colardijn F (1998) The place of intermittent hemodialysis in the treatment of acute renal failure in the ICU patient. Kidney Int Suppl 66:S110–119
Abdeen O, Mehta RL (2002) Dialysis modalities in the intensive care unit. Crit Care Clin 18:223–247
van Bommel EF, Ponssen HH (1997) Intermittent versus continuous treatment for acute renal failure: where do we stand? Am J Kidney Dis 30:S72–79
Gokal R (1993) Quality of life in patients undergoing renal replacement therapy. Kidney Int Suppl 40:S23–27
De Wit GA, Ramsteijn PG, de Charro FT (1998) Economic evaluation of end stage renal disease treatment. Health Policy 44:215–232
Churchill DN, Torrance GW, Taylor DW, Barnes CC, Ludwin D, Shimizu A, Smith EK (1987) Measurement of quality of life in end-stage renal disease: the time trade-off approach. Clin Invest Med 10:14–20
Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM (2005) Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68:2801–2808
Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C (2002) Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis 40:611–622
Manns BJ, Taub KJ, Donaldson C (2000) Economic evaluation and end-stage renal disease: from basics to bedside. Am J Kidney Dis 36:12–28
Hamel MB, Phillips RS, Davis RB, Desbiens N, Connors AF Jr., Teno JM, Wenger N, Lynn J, Wu AW, Fulkerson W, Tsevat J (1997) Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med 127:195–202
SRAU (2005) Renal replacement therapy in Sweden 1991–2004 [in Swedish]. Svenskt Register för Aktiv Uremivård
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bell, M., SWING., Granath, F. et al. Continuous renal replacement therapy is associated with less chronic renal failure than intermittent haemodialysis after acute renal failure. Intensive Care Med 33, 773–780 (2007). https://doi.org/10.1007/s00134-007-0590-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0590-6