Abstract
Aims/hypothesis
An increased risk of type 2 diabetes mellitus is associated with low birthweight after full-term gestation, including amplification of this risk by weight gain during infancy and adult body composition. Premature birth is also associated with insulin resistance, but studies conducted so far have not provided follow-up into adulthood. We studied the effects of (1) lower birthweight (as standard deviation score [SDS]) and infancy weight gain on insulin resistance in 19-year-olds born before 32 weeks of gestation, and (2) the interaction between lower birthweight SDS and infancy weight gain, as well as between lower birthweight and adult body composition, on insulin resistance.
Methods
This was a prospective follow-up study in 346 subjects from the Project on Preterm and Small-for-gestational-age infants cohort, in whom fasting glucose, insulin and C-peptide levels were measured at 19 years. Insulin resistance was calculated with homeostatic modelling (homeostatic model assessment for insulin resistance index [HOMA-IR]).
Results
Birthweight SDS was unrelated to the outcomes. Rapid infancy weight gain until 3 months post-term was weakly associated with higher insulin level (p=0.05). Adult fatness was positively associated with insulin and C-peptide levels and HOMA-IR (all p<0.001). On these parameters, there was a statistical interaction between birthweight SDS and adult fat mass (p=0.002 to 0.03).
Conclusions/interpretation
In subjects born very preterm, rapid infancy weight gain until 3 months predicted higher insulin levels at 19 years, but the association was weak. Adult obesity strongly predicted higher insulin and C-peptide levels as well as HOMA-IR. The effect of adult fat mass on these parameters was dependent on its interaction with birthweight SDS.
Similar content being viewed by others
Introduction
Effects of intrauterine and postnatal growth on the risk, in the general population, of developing type 2 diabetes mellitus are well described. Low birthweight after full-term gestation is associated with insulin resistance, glucose intolerance and type 2 diabetes in later life [1, 2]. The effect of low birthweight on increased type 2 diabetes risk is stronger in subjects who catch up in weight during infancy, and in those who become overweight during childhood and in adult life [3–5]. Also, low weight in infancy has been associated with type 2 diabetes [1, 6].
Less is known about the effects of intrauterine and postnatal growth on insulin resistance in the growing population of survivors of preterm birth. Recently, it was found that 7-year-old children born prematurely were more insulin-resistant than age-matched normal controls [7]. The effect of prematurity was irrespective of intrauterine growth, although another study in 6-year-old preterm offspring found higher basal insulin and C-peptide levels in subjects with birthweights below the 10th percentile [8]. However, both studies were performed in small populations. Very preterm (i.e.<32 weeks of gestation) infants differ from full-term children in postnatal growth pattern, which is characterised by an initial slowing of growth followed by late catch-up growth [9, 10]. To date, one study has focused on insulin resistance in relation to postnatal weight gain after preterm birth [11]. The investigators found that insulin split products were higher in subjects aged 13-16 years with rapid weight gain in the first 2 weeks postnatally. As these three studies were conducted in paediatric populations, it remains uncertain whether the observed associations in childhood persist into adult life. For the same reason, it is unknown whether there is interaction between low birthweight and adult body composition on insulin resistance after very preterm birth.
We provid here a prospective long-term follow-up into adulthood of a well-described cohort of men and women born very preterm, in whom insulin resistance was assessed at the age of 19 years. Within this study population, we tested the effects of lower birthweight for gestational age and rapid infancy weight gain on insulin resistance at the age of 19 years. We also tested whether there was interaction between lower birthweight and rapid infancy weight gain, and between lower birthweight and adult body composition, on insulin resistance.
Subjects, materials and methods
Subjects
The Project on Preterm and Small-for gestational-age infants (POPS) study is a nationwide multicentre prospective follow-up study, which comprises 94% of all liveborn very preterm (<32 weeks of gestation) and/or very low birthweight (<1,500 g) infants born in the Netherlands in 1983, and has documented birth, growth and a number of other characteristics from birth onwards [12, 13]. At follow-up visits at age 3 months and 1 year post-term, weight was recorded. At age 19 years, all 637 alive subjects born with a gestational age below 32 weeks who were free from congenital skeletal deformations, Down’s syndrome, chromosomal abnormalities, multiple congenital deformations or inborn errors of metabolism, and who were not born to mothers with gestational diabetes, were approached by mail to participate in the POPS-19 study. Subjects with diabetes mellitus, or on thyroid hormone or systemic corticosteroids, as well as pregnant women, were excluded. The approval of the medical ethical committees of all participating centres was obtained for the POPS-19 study.
Study protocol
Subjects, who gave written informed consent to participate, were seen after an overnight fast between 0830 and 1000 hours between April 2002 and May 2003 at one of the outpatient clinics of the ten participating centres. Assessors were blinded with respect to the perinatal characteristics of the subjects.
Venous blood was obtained after 30 min in a supine position. Thereafter, anthropometry was performed, for which assessors had received extensive training prior to the study, and re-training during the entire study period at 2-month intervals. Subjects were measured barefoot while wearing underclothing only. Weight was measured to the nearest 0.1 kg on a balance scale, and height to the nearest 0.1 cm with a fixed stadiometer. Waist and hip circumferences were measured at 0.1-cm accuracy using standard methods [14]. Four skinfold thickness measurements were taken in duplicate with a calibrated skinfold calliper on the left side of the body: at triceps, biceps, subscapular and iliacal regions. From these measurements, fat mass and the corresponding fat-free mass were calculated using the equations of Durnin and Rahaman [15]. A more detailed description of skinfold thickness measurements obtained in the POPS-19 study has been published elsewhere [16].
Laboratory analyses
Blood samples were stored at −80°C, and thawed only once immediately before analysis. Glucose was measured in a fully automated computerised laboratory system with an Hitachi 747 (Hitachi, Tokyo, Japan) chemistry analyser, and insulin and C-peptide were measured with highly sensitive RIAs (Linco, St Charles, MO, USA; detection levels 0.1 mU/l and 0.03 mmol/l, respectively; interassay CV 4.7–12.2% and 3.2–9.3% at different levels, respectively). A homeostatic model assessment for insulin resistance index (HOMA-IR) was calculated [17]. Insulin and C-peptide levels, and HOMA-IR were considered as parameters of insulin resistance. Insulin level and HOMA-IR correlate strongly with S i assessed by the frequently sampled IVGTT in young persons [18, 19].
Statistical analysis
Auxological data at birth and on subsequent occasions were converted to standard deviation scores (SDSs), to correct for (gestational) age and sex, using Swedish references for preterm infants [20], and recently collected Dutch references [14, 21, 22], respectively.
Results in Tables 1 and 2 are presented as means±SD, or medians (interquartile range) if variables were not normally distributed (insulin and HOMA-IR). These variables were log-transformed before statistical comparison.
Birthweight is a strong predictor of postnatal size. Therefore, the multivariate linear regression model developed by Li et al. was used to distinguish between the separate effects of birthweight SDS, and of postnatal size (at the age of 3 months, 1 year and 19 years) on the parameters of insulin resistance [23]. First, the effect of birthweight SDS on the parameters of insulin resistance was studied. Subsequently, residual (observed-expected) postnatal size was entered into the model. Expected postnatal size was based upon birthweight SDS only. Hence, residual postnatal size can be interpreted as growing more (or less) than would be expected from a given birthweight SDS. Thereafter, the interaction term (birthweight SDS×residual, with subtraction of means) was entered. Recently [24], the algebraic concept of this model was explained, showing that it can be rewritten to the model by Lucas et al. [25] In the applied model, postnatal size is made statistically unrelated to birthweight SDS.
Analyses with birthweight SDS and infancy weight gain were repeated with adjustment for the possible confounders sex, race (white or non-white), socio-economic status (≤ or >2), multiple pregnancy (singleton or non-singleton), gestational age (≤ or >30 weeks), parity (0 or >0) and hypertension during pregnancy (yes or no). Analyses with adult size and body composition were repeated with adjustment for sex, race and socio-economic status.
Statistical significance was defined as a p value ≤0.05. Non-linear associations were tested by first producing quarters of birthweight SDS and infancy weight gain. These quarters were compared with respect to the parameters of insulin resistance.
Results
At age 19 years, 637 men and women born before 32 weeks of gestation were still alive and eligible for inclusion: 395 consented to participate, whereas 242 refused or were not traceable. Three of the 395 participants met one of the exclusion criteria (one woman was pregnant at the time of assessment, and two subjects used systemic corticosteroids), 27 failed to give blood, and 19 attended not-fasted. Therefore, 346 individuals were included in the statistical analyses.
Non-response was associated with male sex, non-white race and lower socio-economic status (Table 1). It was not associated with gestational age or birthweight. Nineteen individuals (6%) had a birthweight below −2 SDS, 73 (23%) had a weight at 3 months below −2 SDS, and 57 (18%) had a weight at 1 year below −2 SDS.
For both sexes, mean values for height and weight were below the population reference means, while the means for waist circumference and WHR were greater, especially in the women (Table 2). Women had greater absolute and relative fat mass than men. Men had greater fat-free mass and higher glucose levels than women. There was no difference between the sexes for insulin and C-peptide levels and HOMA-IR. Of the women, 121 (67%) used oral contraceptives at the time of assessment, but there were no significant differences in the parameters of insulin resistance between pill and non-pill users (data not shown).
Birthweight SDS was unrelated to the parameters of insulin resistance (Table 3). Rapid infancy weight gain until 3 months was associated with a higher insulin level and HOMA-IR. After correction for possible confounders, the strength of the relationship with HOMA-IR remained unchanged but statistical significance was lost. Rapid infancy weight gain until 1 year was associated only with higher HOMA-IR, which relationship lost statistical significance after correction for possible confounders. No interaction between birthweight SDS and infancy weight gain on the parameters of insulin resistance was observed. There was no evidence for non-linearity in the associations between early growth and the parameters of insulin resistance (data not shown).
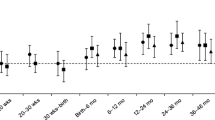
Except for height SDS, all measures of adult size and body composition were strongly associated with insulin and C-peptide levels as well as HOMA-IR (Table 4). Interaction between birthweight SDS and adult absolute and relative fat mass on these parameters was observed. Also, interaction between birthweight SDS and adult height SDS on insulin level and HOMA-IR, and between birthweight SDS and adult waist circumference SDS on HOMA-IR, was found. Fig. 1 displays the direction of the interaction of birthweight SDS and adult absolute fat mass on HOMA-IR, showing that higher fat mass after lower birthweight SDS has larger effects on the parameters of insulin resistance than does higher fat mass after higher birthweight SDS.
Effects of birthweight SDS and adult absolute fat mass on log HOMA-IR at age 19 years. LBW lower birthweight SDS; HBW higher birthweight SDS. HBW-lean birthweight SDS ≥0 and absolute fat mass ≤median for sex (n=78); LBW-lean birthweight SDS<0 and absolute fat mass ≤median for sex (n=90); HBW-obese birthweight SDS ≥0 and absolute fat mass >median for sex (n=85); LBW-obese birthweight SDS<0 and absolute fat mass>median for sex (n=81)
Discussion
From birth onwards we followed a relatively large cohort of subjects born very preterm. At age 19 years, insulin resistance was assessed using fasting levels of insulin and C-peptide as well as HOMA-IR. We found that rapid infancy weight gain until 3 months predicted higher insulin levels at age 19 years, but the association was weak. Adult fat accumulation strongly predicted higher insulin and C-peptide levels as well as higher HOMA-IR at age 19 years. The effect of adult fat accumulation on these parameters of insulin resistance was dependent on its interaction with birthweight SDS. This was most clearly the case for increased fat mass per se, rather than for more abdominal fat. However, it should be emphasised that the typical centralisation of fat distribution occurs with advancing age so that such relationships may become more clear later in adulthood.
It has been suggested that the association between early growth and type 2 diabetes risk is the result of a biological phenomenon which has been called fetal or perinatal programming [26]. Throughout the years, several hypotheses based on the concept of programming have been proposed to underlie this association, including the thrifty phenotype, fetal salvage, catch-up growth and stem-cell hypotheses [27–30]. There are also indications that the association is not the result of programming but of confounding (especially by socio-economic status), selective survival or response [31, 32], or genes that affect both the path of early growth and insulin resistance [33]. Despite incomplete follow-up, our participants did not significantly differ from non-respondents in perinatal characteristics. Non-response was higher among men, non-whites and those with lower socio-economic status. As expected, we found that subjects with lower socio-economic status had higher parameters of insulin resistance than those with higher socio-economic status, but the difference was not statistically significant. We also found that whites were as equally insulin resistant as the rather heterogeneous group of non-whites, including 11 subjects of Mediterranean origin, ten Africans, 15 Asians and four others (data not shown), while it is known that prevalence rates of type 2 diabetes are substantially higher among blacks and Indians. The question arises whether selective response could account for (part of) the observed associations in our study sample. Statistical adjustment for a number of variables, including socio-economic status and race, in regression analyses hardly changed any of the coefficients between early growth and the parameters of insulin resistance. This makes bias introduced by selective response less likely, although it does not exclude the possibility completely. A possible relationship between birthweight SDS and the parameters of insulin resistance would be concealed if the low birthweight SDS subjects with a high risk of insulin resistance selectively declined to participate. Furthermore, the relationship between infancy weight gain and insulin level would be artificial if the slow weight gain subjects with a high risk of insulin resistance selectively refused to participate. This seems not very likely. Moreover, although non-response was associated with demographic factors linked to insulin resistance, in the entire birth cohort (n=1,012) there were no effects of race or socio-economic status on birthweight SDS or infancy weight gain.
Although the majority of full-term small-for-gestational-age (SGA) babies show catch-up growth after birth [34], infancy weight gain of our study population was slow, which is reflected by the low mean weight SDS at 3 months and 1 year. However, in contrast to full-term SGA babies, many very preterm infants suffer from life-threatening conditions after birth which require a shift in energy expenditure, enabling them to survive at the expense of somatic growth; e.g. by respiratory distress necessitating assisted ventilation, or infections.
To date, a number of studies have investigated the effect of infancy weight gain on later type 2 diabetes [3, 11, 35, 36]. In middle-aged subjects, low weight at birth and at age 1 year were associated with type 2 diabetes, but the rate of weight gain in the first year of life was unrelated to type 2 diabetes [35]. However, in 1-year-old infants born SGA, catch-up growth in weight until age 1 year was associated with higher fasting insulin levels [3]. Similarly, in children aged 8 years, rapid weight gain between birth and age 3 years was related to insulin resistance [36]. Also in prematurely born subjects aged 13–16 years, effects of early postnatal weight gain on later insulin resistance have been reported. Boys and girls with rapid weight gain in the first 2 weeks postnatally had higher concentrations of proinsulin and 32–33 split proinsulin [11]. Our findings in young adults born very preterm support these previous observations in paediatric populations.
We found that lower birthweight SDS strongly modified the effect of greater absolute and relative fat mass on insulin and C-peptide levels as well as HOMA-IR in young adulthood, which is consistent with previous studies in full-term 20-year-old subjects [5]. We also found an interaction effect between lower birthweight SDS and greater adult height SDS on insulin level and HOMA-IR, but this probably reflects the effect of greater absolute fat mass (which is closely related to height). The interaction between lower birthweight SDS and greater adult relative fat mass (which is independent of height) on the parameters of insulin resistance suggests an effect of adult fat accumulation rather than of height.
Conclusions and implications
In conclusion, our study in a relatively large cohort of men and women born very preterm showed that rapid infancy weight gain until 3 months predicted higher insulin levels at age 19 years, but the association was weak. Adult fat accumulation strongly predicted higher insulin and C-peptide levels as well as higher HOMA-IR at age 19 years. The effect of adult fat accumulation on these parameters of insulin resistance was dependent on its interaction with birthweight SDS.
A previous study in the same cohort showed that rapid infancy weight gain was a strong predictor of adult fat accumulation [16], whereas this study showed only a weak effect of rapid infancy weight gain until 3 months on insulin level (and no effect on C-peptide level and HOMA-IR) in young adulthood. At present, it is unclear whether interventions aimed at discouraging infancy weight gain would improve adult body composition and reduce the chance of developing type 2 diabetes. However, recent evidence suggests that rapid infancy weight gain of very preterm infants is beneficial for several neurodevelopmental outcomes [37, 38], making intervention (i.e. undernutrition) hard to justify.
Recently, it was found that the survivors of very preterm birth are already more insulin resistant at the age of 7 years [7]. In line with these observations, we found that HOMA-IR (which normally approximates to 1 in young non-obese persons, if glucose is measured in mmol/l and insulin in mU/l [17]) was relatively high in our study population. Also, we found that our subjects had already some centralisation of fat distribution compared with population references. As there is strong tracking of obesity from childhood to young adulthood [39], we would like to call upon paediatricians and primary healthcare workers (1) to be alert to fat accumulation during childhood in the follow-up of very preterm subjects, especially of those born SGA, and (2) to intervene, even though it has not yet been tested whether weight reduction in very preterm subjects can reverse insulin resistance. The question of whether the survivors of very preterm birth, and especially those born SGA who subsequently become overweight, have a premature onset of type 2 diabetes remains very interesting and should be addressed.
Abbreviations
- HOMA-IR:
-
homeostatic model assessment for insulin resistance index
- POPS:
-
Project on Preterm and Small-for-gestational-age infants
- SDS:
-
standard deviation score
- SGA:
-
small-for-gestational-age
References
Hales CN, Barker DJ, Clark PM et al (1991) Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022
Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM (2003) Is birth weight related to later glucose and insulin metabolism? A systematic review. Diabet Med 20:339–348
Soto N, Bazaes RA, Pena V et al (2003) Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab 88:3645–3650
Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D (2000) The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 133:176–182
Levitt NS, Lambert EV, Woods D, Seckl JR, Hales CN (2005) Adult BMI and fat distribution but not height amplify the effect of low birthweight on insulin resistance and increased blood pressure in 20-year-old South Africans. Diabetologia 48:1118–1125
Bhargava SK, Sachdev HS, Fall CH et al (2004) Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 350:865–875
Hofman PL, Regan F, Jackson WE et al (2004) Premature birth and later insulin resistance. N Engl J Med 351:2179–2186
Bazaes RA, Alegria A, Pittaluga E, Avila A, Iniguez G, Mericq V (2004) Determinants of insulin sensitivity and secretion in very-low-birth-weight children. J Clin Endocrinol Metab 89:1267–1272
Niklasson A, Engstrom E, Hard AL, Albertsson-Wikland K, Hellstrom A (2003) Growth in very preterm children: a longitudinal study. Pediatr Res 54:899–905
Knops NB, Sneeuw KC, Brand R et al (2005) Catch-up growth up to ten years of age in children born very preterm or with very low birth weight. BMC Pediatr 5:26
Singhal A, Fewtrell M, Cole TJ, Lucas A (2003) Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 361:1089–1097
Verloove-Vanhorick SP, Verwey RA, Brand R, Gravenhorst JB, Keirse MJ, Ruys JH (1986) Neonatal mortality risk in relation to gestational age and birthweight. Results of a national survey of preterm and very-low-birthweight infants in the Netherlands. Lancet 1:55–57
Walther FJ, den Ouden AL, Verloove-Vanhorick SP (2000) Looking back in time: outcome of a national cohort of very preterm infants born in The Netherlands in 1983. Early Hum Dev 59:175–191
Fredriks AM, van Buuren S, Fekkes M, Verloove-Vanhorick SP, Wit JM (2005) Are age references for waist circumference, hip circumference and waist-hip ratio in Dutch children useful in clinical practice? Eur J Pediatr 164:216–222
Durnin JV, Rahaman MM (1967) The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr 21:681–689
Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, Wit JM, Dekker FW (2005) Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr 81:480–487
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Gungor N, Saad R, Janosky J, Arslanian S (2004) Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr 144:47–55
Conwell LS, Trost SG, Brown WJ, Batch JA (2004) Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 27:314–319
Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P (1991) An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr Scand 80:756–762
Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP (2000) Body index measurements in 1996-1997 compared with 1980. Arch Dis Child 82:107–112
Fredriks AM, van Buuren S, Burgmeijer RJ et al (2000) Continuing positive secular growth change in The Netherlands 1955–1997. Pediatr Res 47:316–323
Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R (2003) Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr 77:1498–1505
Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, van Houwelingen HC (2005) A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol 58:1320–1324
Lucas A, Fewtrell MS, Cole TJ (1999) Fetal origins of adult disease—the hypothesis revisited. BMJ 323:572–573
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet 341:938–941
Hales CN, Barker DJ (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601
Hofman PL, Cutfield WS, Robinson EM et al (1997) Insulin resistance in short children with intrauterine growth retardation. J Clin Endocrinol Metab 82:402–406
Cianfarani S, Germani D, Branca F (1999) Low birthweight and adult insulin resistance: the ‘catch-up growth’ hypothesis. Arch Dis Child Fetal Neonatal Ed 81:F71–F73
Cianfarani S (2003) Foetal origins of adult diseases: just a matter of stem cell number? Med Hypotheses 61:401–404
Kramer MS (2000) Invited commentary: association between restricted fetal growth and adult chronic disease: is it causal? Is it important? Am J Epidemiol 152:605–608
Paneth N, Susser M (1995) Early origin of coronary heart disease (the ‘Barker hypothesis’). BMJ 310:411–412
Hattersley AT, Tooke JE (1999) The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 353:1789–1792
Albertsson-Wikland K, Wennergren G, Wennergren M, Vilbergsson G, Rosberg S (1993) Longitudinal follow-up of growth in children born small for gestational age. Acta Paediatr 82:438–443
Eriksson JG, Forsen TJ, Osmond C, Barker DJ (2003) Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care 26:3006–3010
Ong KK, Petry CJ, Emmett PM et al (2004) Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 47:1064–1070
Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T (2003) Intellectual and psychological performance in males born small for gestational age. Horm Res 59 (Suppl 1):139–141
Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH (2003) Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr 143:163–170
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH (1997) Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337:869–873
Acknowledgements
This specific part of the POPS-19 study was supported by a grant from the Netherlands Organisation for Scientific Research (NWO). The POPS-19 study was supported by grants from the Netherlands Organisation for Health Research and Development (ZonMw), Edgar Doncker Foundation, Foundation for Public Health Fundraising Campaigns, Phelps Foundation, Swart-van Essen Foundation, Foundation for Children’s Welfare Stamps, TNO Quality of Life, Netherlands Organisation for Scientific Research (NWO), Dutch Kidney Foundation, Sophia Foundation for Medical Research, Stichting Astmabestrijding, and the Royal Effatha Guyot group.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
For a list of participants in the Dutch POPS-19 Collaborative Study Group, see Appendix
Appendix
Appendix
Participants in the Dutch POPS-19 Collaborative Study Group are: TNO Quality of Life, Leiden (E. T. M. Hille, C. H. de Groot, H. Kloosterboer-Boerrigter, A. L. den Ouden, A. Rijpstra, S. P. Verloove-Vanhorick, J. A. Vogelaar); Emma Children’s Hospital AMC, Amsterdam (J. H. Kok, A. Ilsen, M. van der Lans, W. J. C. Boelen-van der Loo, T. Lundqvist, H. S. A Heymans); University Hospital Groningen, Beatrix Children’s Hospital, Groningen (E. J. Duiverman, W. B. Geven, M. L. Duiverman, L. I. Geven, E. J. L. E. Vrijlandt); University Hospital Maastricht, Maastricht (A. L. M. Mulder, A. Gerver); University Medical Center St Radboud, Nijmegen (L. A. A. Kollée, L. Reijmers, R. Sonnemans); Leiden University Medical Center, Leiden (J. M. Wit, F. W. Dekker, M. J. J. Finken); Erasmus MC-Sophia Children’s Hospital, University Medical Center Rotterdam (N. Weisglas-Kuperus, M. G. Keijzer-Veen, A. J. van der Heijden, J. B. van Goudoever); V. U. University Medical Center, Amsterdam (M. M. van Weissenbruch, A. Cranendonk, H. A. Delemarre-van de Waal, L. de Groot, J. F. Samsom); Wilhelmina Children’s Hospital, UMC, Utrecht (L. S. de Vries, K. J. Rademaker, E. Moerman, M. Voogsgeerd); Máxima Medical Center, Veldhoven (M. J. K. de Kleine, P. Andriessen, C. C. M. Dielissen-van Helvoirt, I. Mohamed); Isala Clinics, Zwolle (H. L. M. van Straaten, W. Baerts, G. W. Veneklaas Slots-Kloosterboer, E. M. J. Tuller-Pikkemaat); Royal Effatha Guyot Group, Zoetermeer (M. H. Ens-Dokkum); Association for Parents of Premature Babies (G. J. van Steenbrugge).
Rights and permissions
About this article
Cite this article
Finken, M.J.J., Keijzer-Veen, M.G., Dekker, F.W. et al. Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia 49, 478–485 (2006). https://doi.org/10.1007/s00125-005-0118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-0118-y