Abstract
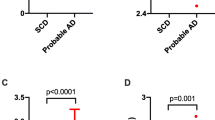
Cerebrospinal fluid (CSF) biochemical markers for Alzheimer disease (AD) would be of great value to improve the clinical diagnostic accuracy of the disorder. As abnormally phosphorylated forms of the microtubule-associated protein tau have been consistently found in the brains of AD patients, and since tau can be detected in CSF, two assays based on several well-defined monoclonal tau antibodies were used to study these proteins in CSF. One assay detects most normal and abnormal forms of tau (CSF-tau), while the other is highly specific for phosphorylated tau (CSF-PHFtau). A marked increase in CSF-PHFtau was found in AD (2230±930 pg/mL), as compared with controls (640±230 pg/mL;p<0.0001), vascular dementia, VAD (1610±840 pg/mL;p<0.05), frontal lobe dementia, FLD (1530±1000 pg/mL;p<0.05), Parkinson disease, PD (720±590 pg/mL;p<0.0001), and patients with major depression (230±130 pg/mL;p<0.0001). Parallel results were obtained for CSF-tau. No less than 35/40 (88%) of AD patients had a CSF-PHFtau value higher than the cutoff level of 1140 pg/mL in controls. The present study demonstrates that elevated tau/PHFtau levels are consistently found in CSF of AD patients. However, a considerable overlap is still present with other forms of dementia, both VAD and FLD. CSF-tau and CSF-PHFtau may therefore be useful as a positive biochemical marker, to discriminate AD from normal aging, PD, and depressive pseudodementia. Further studies are needed to clarify the sensitivity and specificity of these assays, including follow-up studies with neuropathological examinations.
Similar content being viewed by others
References
American Psychiatric Association (1987)Diagnostic and Statistical Manual of Mental Disorders, 3rd ed., American Psychiatric Association, Washington, DC.
Bancher, C., Brunner C., Lassman H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., and Wisniewski H. M. (1989) Accumulation of abornmally phosphorylated τ precedes the formation of neurofibrillary tangles in Alzheimer disease.Brain Res. 477, 90–99.
Bissette G., Smith W. H., Dole K. C., Crain B., Ghanbari H., Miller B., and Nemeroff C. B. (1991) Alterations in Alzheimer’s disease-associated protein in Alzheimer’s disease frontal and temporal cortex.Arch. Gen. Psychiat. 48, 1009–1012.
Blennow K., Wallin A., and Gottfries C. G. (1991) Presence of parietal lobe symptomatology distinguishes early and late onset Alzheimer’s disease.Int. J. Geriatr. Psychiat.,6, 147–154.
Blennow K. and Wallin A. (1992) Clinical heterogeneity of probable Alzheimer’s disease.J. Geriatr. Psychiatry Neurol. 5, 106–113.
Blennow K., Fredman P., Wallin A., Gottfries C. G., Langstrom L., and Svennerholm L. (1993) Protein analyses in cerebrospinal fluid: I. Influence of concentration gradients for proteins on cerebrospinal fluid/serum albumin ratio.Eur. Neurol. 33, 126–128.
Bramblett G. T., Trojanowski J. Q., and Lee V. M. Y. (1992) Regions with abundant neurofibrillary pathology in human brain exhibit a selective reduction in levels of binding-competent τ and accumulation of abnormal τ-isoforms (A68 proteins).Lab. Invest. 66, 212–222.
Coleman P. D. and Flood D. G. (1987) Neuron numbers and dendrite extent in normal aging and Alzheimer’s disease.Neurobiol. Aging 8, 521–545.
Davies C. A., Mann D. M. A., Sumpter P. Q., and Yates P. O. (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease.J. Neurol. Sci. 78, 151–164.
Delacourte A., Flament S., Dibe E. M., Hublau P., Sablonniére B., Hémon B., Shérrer V., and Défossez A. (1990) Pathological proteins tau 64 and 69 are specifically expressed in the somatodendritic domain of the degenerating cortical neurons during Alzheimer’s disease.Acta Neuropathol. 80, 111–117.
Dewar D., Graham D. I., Teasdale G. M., and McCulloch J. (1994) Cerebral ischemia induces alterations in tau and ubiquitin proteins.Dementia 5, 168–173.
Folstein M., Folstein S., and McHugh P. (1975) “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician.J. Psychiatr. Res. 12, 189–198.
Garver T. D., Harris K. A., Lehman R. A. W., Lee V. M. Y., Trojanowski J. Q., and Billingsley M. L. (1994) τ phosphorylation in human, primate, and rat brain: evidence that a pool of τ is highly phosphorylated in vivo and is rapidly dephosphorylated in vitro.J. Neurochem. 63, 2279–2287.
Ghanbari H. A., Kozuk T., Miller B. E., and Riesing S. (1990) A sandwich enzyme immunoassay for detecting and measuring Alzheimer’s disease-associated proteins in human brain tissue.J. Clin. Lab. Anal. 4, 189–192.
Goedert M., Wischik C. M., Crowther R. A., Walker J. E., and Klug A. (1988) Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau.Proc. Natl. Acad. Sci. USA 85, 4051–4055.
Goedert M., Spillantini M., and Jakes R. (1991) Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau.Neurosci. Lett. 126, 149–154.
Goedert M. (1993) Tau protein and the neurofibrillary pathology of Alzheimer’s disease.Trends Neurosci. 16, 460–465.
Goedert M., Jakes R., Crowther A., Cohen P., Vanmechelen E., Vandermeeren M., and Cras P. (1994) Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: identification of phosphorylation sites in tau protein.Biochem. J. 301, 871–877.
Greenberg S. G. and Davies P. (1990) A preparation of Alzheimer paired helical filaments that displays distinct → proteins by polyacrylamide gel electrophoresis.Proc. Natl. Acad. Sci. USA 87, 5827–5831.
Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., and Binder L. I. (1986) Abnormal phosphorylation of the microtubule-associated protein → (tau) in Alzheimer cytoskeletal pathology.Proc. Natl. Acad. Sci. USA 83, 4913–4917.
Gustafson L. (1987) Frontal lobe degeneration of non-Alzheimer type. 2. Clinical picture and differential diagnosis.Arch. Gerontol. Geriatr. 6, 209–223.
Hamos J. E., DeGennaro L. J., and Drachman D. A. (1989) Synaptic loss in Alzheimer’s disease and other dementias.Neurology 39, 355–361.
Harrington C. R., Mukeatova-Ladinska E. B., Hills R., Edwards P. C., de Garcini E. M., Novak M., and Wischik C. M. (1991) Measurement of distinct immunochemical presentations of tau protein in Alzheimer disease.Proc. Natl. Acad. Sci. USA 88, 5842–5846.
Harrington C. R., Perry R. H., Perry E. K., Hurt J., McKeith I. G., Roth M., and Wischik C. M. (1994) Senile dementia of Lewy Body-type and Alzheimer type are biochemically distinct in terms of paired helical filaments and hyperphosphorylated tau protein.Dementia 5, 215–218.
Hyman B. T., van Hoesen G. W., Wolozin B. L., Davies P., Kromer L. J., and Damosio A. R. (1988) Alz-50 antibody recognizes Alzheimer-related neuronal changes.Ann. Neurol. 23, 371–379.
Ihara Y., Nukina N., Miura R., and Ogawara M. (1986) Phosphorylated tauprotein is integrated into paired helical filaments in Alzheimer’s disease.J. Biochem. 99, 1807–1810.
International Federation of Clinical Chemistry (IFCC) (1987) Approved recommentation on the theory of reference values, Part 5. Statistical treatment of collected reference values. Determination of reference limits.Clin. Chim. Acta 170, 13–32.
Katzman R. (1986) Alzheimer’s disease.N. Engl. J. Med. 314, 964–973.
Khatoon S., Grundke-Iqbal I., and Iqbal K. (1992) Brain levels of microtubule-associated protein → are elevated in Alzheimer’s disease: a radioimmunoslot-blot assay for nanograms of the protein.J. Neurochem. 59, 750–753.
Langston J. W., Widner H., Goetz C. G., Brooks D., Fahn S., Freeman T., and Watts R. (1992) Core assessment program for intracerebral transplantations (CAPIT).Mov. Disord. 7, 2–13.
Lishman W. A. (1987)Organic Psychiatry: the Psychological Consequences of Cerebral Disorder. 2nd ed. Chicago, Year Book Medical Publishers.
Matsuo E., Shin R.-W., Billingsley M. L., Van de Voorde A., O’Connor M., Trojanowski J., and Lee V.-M. (1994) Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau.Neuron 13, 989–1002.
McKhann G., Drachman D., Folstein M., Katzman R., Price D., and Stadlan E. M. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer’s disease.Neurology 34, 939–944.
Mehta P. H., Thal L., and Wisniewski H. M., et al. (1985) Paired helical filaments antigen in CSF. (letter)Lancet II 35.
Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., van der Voorde A., Martin J. J., and Gheuens J. (1992) Monoclonal antibodies with selective specificity for Alzheimer tau are directed against phosphatase-sensitive epitopes.Acta Neuropathol. 84, 265–272.
Mori H., Kondo J., and Ihara Y. (1987) Ubiquitin is a component of paired helical filaments in Alzheimer’s disease.Science 235, 1641–1644.
Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Titani K., and Ihara Y. (1995) Proline-directed and non-proline-directed phosphorylation of PHF-tau.J. Biol. Chem. 270, 823–829.
Mukaetova-Ladinska E. B., Harrington C. R., Hills R., O’Sullivan A., Roth M., and Wischik C. M. (1992) Regional distribution of paired helical filaments and normal tau proteins in aging and in Alzheimer’s disease with and without temporal lobe involvement.Dementia 3, 61–69.
Oyama F., Shimada H., Oyama R., Titani K., and Ihara Y. (1991) Differential expression of β amyloid protein precursor (APP) and tau mRNA in the aged human brain: individual variability and correlation between APP-751 and four-repeat tau.J. Neuropathol. Exp. Neurol. 50, 560–578.
Perry G., Friedman R., Shaw G., and Chau V. (1987) Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains.Proc. Natl. Acad. Sci. USA 84, 3033–3036.
Scheltens P., Vermersch P., and Leys D. (1993) Hétérogénéité de la maladie d’Alzheimer.Rev. Neurol. (Paris) 149, 14–25.
Sjögren T., Sjögren H., and Lindgren Å. (1952) Morbus Alzheimer and morbus Pick: A genetic, clinical and patho-anatomical study.Acta Psych. Neurol. Scand. (Suppl. 82) 66–115.
Szendrei G., Lee V.-M., and Otvos L. (1993) Recognition of the minimal epitope of monoclonol antibody Tau-1 depends upon the presence of a phosphate group but not its localization.J. Neurosci. Res. 34, 243–249.
Thompson E. J. (1988)The CSF Proteins: A Biomedical Approach. Elsevier, Amsterdam, pp. 9–26.
Tibbling G., Link H., and Öhman S. (1977) Principles of albumin and IgG analysis in neurological disorders. I. Establishment of reference values.Scand. J. Clin. Lab. Invest. 37, 385–390.
Tomlinson B. E., Blessed G., and Roth M. (1968) Observations of the brans of non-demented old people.J. Neurol. Sci. 7, 331–356.
Tomlinson B. E., Blessed G., and Roth M. (1968) Observations of the brains of demented old people.J. Neurol. Sci. 11, 205–242.
Tomlinson B. E. and Corsellis J. A. N. (1984)Ageing and the dementias, inGreenfield’s Neuropathology (Hume Adams J., Corsellis J. A. N., and Duchen L. W., eds., Edward Arnold, London, pp. 951–1025.
Vandermeeren M., Mercken M., Vanmechelen E., Six J., Van de Voorde A., Martin J. J., and Cras P. (1993) Detection of → proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzymelinked immunosorbent assay.J. Neurochem. 61, 1828–1834.
Wallin A., Gottfries C. G., Karlsson I., and Svennerholm L. (1989) Decreased myelin lipids in Alzheimer’s disease and vascular dementia.Acta Neurol. Scand. 80, 319–323.
Wallin A. and Blennow K. (1991) The pathogenetic basis of vascular dementia.Alzheimer Dis. Assoc. Disord. 5, 91–102.
Wallin A., Blennow K., and Scheltens P. (1994) Research criteria for clinical diagnosis of “pure” Alzheimer’s disease.Drugs Today 30, 265–273.
Wang G. P., Iqbal K., Bucht G., Winblad B., Wisniewski H. M., and Grundke-Iqbal I. (1991) Alzheimer’s disease: paired helical filament immunoreactivity in cerebrospinal fluid.Acta Neuropathol. (Berl.) 82, 6–12.
Wisniewski K., George A. J., Moretz R. C., and Wisniewski H. M. (1979) Alzheimer neurofibrillary tangles in diseases other than senile and presenile dementia.Ann. Neurol. 5, 288–294.
Wolozin B. and Davies P. (1987) Alzheimer-related neuronal protein A68: specificity and distribution.Ann. Neurol. 22, 521–526.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blennow, K., Wallin, A., Ågren, H. et al. tau protein in cerebrospinal fluid. Molecular and Chemical Neuropathology 26, 231–245 (1995). https://doi.org/10.1007/BF02815140
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02815140