Article Text
Abstract
Introduction Maintaining high levels of physical activity helps to maintain and improve physical health and quality of life, and plays a role in reducing adverse effects due to cancer treatments. Moreover, a greater degree of cardiorespiratory fitness is associated with reduced risk of all-cause mortality. However, there are no home-based programme for improving cardiorespiratory fitness using body weight exercises for breast cancer survivors. This study will assess the efficacy of the newly developed habit-B programme on maximum oxygen uptake compared with treatment as usual with wearable device. The effects of this programme on exercise habits, level of physical activity, physical fitness and subjective indices will also be investigated.
Methods and analysis This is a 12-week, parallel-group, single-blind, randomised controlled trial. Allocation will be managed by a central server using a computer-generated random allocation sequence provided by an independent data centre. Participants will be assigned to the habit-B programme (high-intensity interval training, exercise counselling + guidance, home-based exercise support using information and communication technology, and a wearable device) or treatment as usual with a wearable device. Subjects will be sedentary women aged 20–59 years who have received breast surgery in the past 2–13 months after the diagnosis of invasive breast cancer (stages I–IIa) and have never received chemotherapy except for hormone therapy. The primary endpoint is the change in peak oxygen uptake (VO2peak; mL/kg/min) between the groups after 12 weeks of intervention.
Ethics and dissemination The study protocol was approved by the Institutional Review Board of the National Cancer Center Japan on 28 February 2019 (ID: 2018-347). The findings will be disseminated through peer-reviewed publications and conference presentations.
Trial registration number UMIN000036400.
- breast tumours
- sports medicine
- preventive medicine
- clinical physiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This clinical trial will be the world’s first home-based high-intensity interval training programme using information and communication technology to increase cardiorespiratory fitness in sedentary breast cancer survivors.
The habit-B programme is designed based on the theory of Bandura for sedentary breast cancer survivors to develop exercise habits.
Collaborative work with professionals in the field of clinical oncology, exercise science, mental health, rehabilitation, public health, nursing and cancer survivors constitutes a new model for cancer survivorship care.
We cannot rigorously exclude breast cancer survivors who may have exercise habits because the definition of sedentary subjects will be based on self-report.
Introduction
Breast cancer, which is one of the most common cancers affecting women, newly affects over 86 000 women each year.1 It is recommended that cancer survivors acquire and maintain healthy behaviours to extend survival, such as staying reasonably fit through appropriate physical exercise and having a healthy diet.2 High levels of physical activity help to maintain and improve physical health and quality of life (QOL), and play a role in reducing short-term and long-term side effects of cancer treatment. The American Cancer Society guidelines recommend a minimum of 150 min moderate physical activity or 75 min intensive physical activity per week, in addition to usual physical activity in daily life,3 and the Japan Breast Cancer Society’s Clinical Guidelines for Breast Cancer strongly recommend maintaining a high level of physical activity.4 5
The Exercise and Nutrition to Enhance Recovery and Good Health for You Trial, an early and representative study conducted in the USA, demonstrated the effectiveness of a home exercise programme and nutritional advice to promote weight loss among breast cancer survivors.6 However, because the proportion of overweight adults in Japan is lower than that in the USA,7 it is not appropriate to adopt the US-developed programme in Japan. It would appear suitable though to develop intervention around measurements of VO2peak, the globally important health indicator of cardiorespiratory fitness.8 9
VO2peak decreases with age, and a meta-analysis has suggested that women who are sedentary have a decrease of 3.2 mL/kg/min over 10 years10. A cohort study suggested that the all-cause mortality rate decreased by 17% for each 1 metabolic equivalent (MET) increase (3.5 mL/kg/min) in VO2peak for women.11 A systematic review demonstrated that the median VO2peak of breast cancer survivors not receiving chemotherapy was lower than that of healthy sedentary women (25.3 mL/kg/min vs 29.7 mL/kg/min).12 In a 16-year follow-up study of breast cancer survivors,12 HRs were 0.67 for the moderate VO2peak group (8.5 METs) and 0.45 for the high group (11.1 METs) compared with the low VO2peak group (6.7 METs). In this report, the mortality rate was reduced by maintaining a VO2peak of 8 METs or above.12 Against this background, it is necessary to develop an exercise programme that will increase VO2peak in breast cancer survivors.
When considering how to increase VO2peak, research has advanced in recent years with the use of high-intensity interval training (HIIT) for athletes and sedentary populations.13–15 HIIT allows subjects to exercise at vigorous intensity within 10 min, resulting in improved cardiorespiratory fitness.9 16 A recent systematic review showed, based on the findings of 12 studies of HIIT conducted in supervised experimental settings, that HIIT appeared to be more beneficial than treatment as usual for improving physical fitness and health-related outcomes in cancer survivors during any stage of treatment and aftercare.16 To our knowledge, only one study has evaluated changes in cardiorespiratory fitness following a home-based HIIT programme, which involved healthy young women.17 Given that no home-based HIIT programme with body weight exercises has been reported for breast cancer survivors to date, it is necessary to develop such programme and examine their efficacy and feasibility.
Recent emerging technologies such as smartphone applications (apps) and wearable devices are promising tools for the monitoring of daily activity levels of patients with cancer and for facilitating coaching, self-monitoring, feedback and encouragement to exercise.18 19 Apps20 and wearable devices21 are being used for both subjective and objective measures in the field of clinical oncology. However, further investigations are needed of their potential utility in objective evaluations of physical activity as well as in lifestyle modification and maintenance and enhancement of QOL in real-world settings.
The aim of this study is to investigate the effect of the newly developed habit-B programme, comprising home-based high-intensity interval training and behavioural modification using information and communication technology on cardiorespiratory fitness and exercise habits for sedentary breast cancer survivors. We will investigate whether the habit-B programme improves VO2peak compared with a control group as well as investigate the safety and feasibility of programme. The secondary objectives are to investigate the effect of this programme on exercise habits, physical activity level, physical function and subjective measures.
Methods and analysis
Trial design
In this study of a 12-week, parallel-group, single-blind, randomised controlled trial (figure 1), participants will be randomly assigned to intervention either with the habit-B programme (HIIT exercise, exercise counselling +guidance, home-based exercise support using information and communication technology (ICT) and a wearable device) or treatment as usual with a wearable device (Fitbit Versa smart watch; Fitbit, San Francisco, California). An independent data centre will provide computer-generated random allocation. Participants will be assigned by the minimisation method, a form of dynamic randomisation, using two prognostic factors: VO2peak and age. Based on the allocation sequences, the contents of the app that participants use during the trial will be assigned automatically to either the habit-B programme or control.
Flow diagram of study participants. HIIT, high-intensity interval training; ICT, information and communication technology.
Participants
The eligibility criteria for participants are as follows: (1) female, aged between 20 and 59 years at diagnosis (stages I–IIa); (2) diagnosed with invasive breast cancer within 2–13 months after surgery; (3) not requiring cancer chemotherapy aside from hormone therapy; (4) currently engages in not more than moderate intensity exercise for 30 min on two separate days per week (total of 60 min), which is based on the National Health and Nutrition Survey Japan22; (5) ability to complete an electronic Patient Reported Outcome (e-PRO) Questionnaire via a smartphone; (6) consent to trial participation obtained in writing from the patient themselves and (7) ability to read, write and understand Japanese. The exclusion criteria are as follows: (1) judged to have severely reduced cognitive function by a primary physician; (2) exercise judged to be risky by a primary physician; (3) history of smoking within the previous 12 months; (4) body mass index of 30 or above; (5) abnormal ECG in preoperative testing, resting heart rate (HR) below 50 beats/min or above 100 beats/min, or stage III hypertension or above (diastolic blood pressure over 110 mm Hg or systolic blood pressure over 180 mm Hg) and (6) judged unfit for the trial by a primary physician for other reasons such as the administration of beta-adrenergic blocking agents.
Interventions
This will be a randomised, single-blind study. Participants will be enrolled and assigned using an electronic data capture system with an app. The protocol intervention will be started within 21 days of enrolment. If for some reason the start is delayed beyond 21 days, the reason will be entered into the electronic Case Report Form (eCRF). If it is determined that the intervention cannot be started, the details will be noted in the eCRF as ‘protocol intervention stopped’.
habit-B programme group
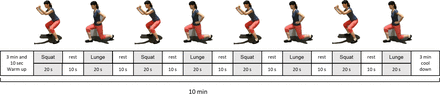
The habit-B programme (figure 2) comprises home-based exercise support using 6 weeks of exercise counselling + exercise guidance (once per week, 6 times total, 30 min per session) and 12 weeks of ICT interventions, which are provided with personalised email (once per week, 12 times in total), and a newly developed exercise app (during each exercise session, shown in figure 3). Participants will be encouraged to complete the following programme HIIT using specific body weight exercises for one 10 min training session 3 times a week for 12 weeks (total of 36 sessions during the trial period, as shown in figures 2 and 4): (1) one bout of 10 min exercises; (2) a total of 10 min exercise, comprising a 3 min warm-up, 4 min training (8 sets of 20 s exercise +10 s rest), and a 3 min cool-down; (3) these training exercises are designed to increase in intensity each week; and (4) the details of these exercises are divided into three stages according to cardiorespiratory fitness (VO2peak) at week 0 and the contents of training are designed to increase physical strength incrementally in accordance with the individual’s level of strength. In addition, we will continue to follow-up on the between-group differences in e-PRO data of change from baseline to 24 and 36 weeks post intervention.
Study design of the habit-b programme (home-based high-intensity interval training and behavioural modification using information and communication technology on cardiorespiratory fitness and exercise habits for sedentary breast cancer survivors). HIIT, high-intensity interval training; ICT, information and communication technology.
Screenshots of the application for the smartphone-based exercise movie and exercise record.
Sample of high-intensity interval training using body weight exercise.
The social cognitive theory proposed by Bandura et al 23 may be helpful to apply when considering interventions for behavioural change, chiefly in that it affords a framework for understanding the underlying reasons for making and maintaining health behaviours.24 Its key concepts involve (1) understanding of health risks and benefits, (2) perceived self-efficacy of being able to control one’s own health habits, (3) projected costs and benefits or expected outcomes, (4) specified health goals with short-term and long-term intentions to engage in the behaviour, (5) perceived facilitators and social support and (6) barriers to instituting change.23 24 In the present study, we developed the exercise programme applying the first five concepts.
We will also provide the participant a brochure as a reminder to exercise and will check the type of physical activity they engage in in daily life (figure 5). When developing the habit-B programme, we collaborated closely with exercise scientists, oncologists, physical therapists, nurses, mental health practitioners and breast cancer survivors.
Brochure reminding participants about the importance of exercise and providing instructions of exercise. NCC, National Cancer Center.
Control group
The control group will be provided a wearable device and set-up support at the start of intervention. Self-monitoring using the wearable device will be recommended during the trial period. While there are no reports of increasing VO2peak with the use of a wearable device alone, other reports have ruled out their efficacy with regard to effects on exercise habits.25 In this study, the wearable devices are used only to monitor physical activity in the control group.
Procedure
Data collection, management, monitoring and auditing
We will collect all data except qualitative interview data, blood samples, faecal samples and medical economic costs through the e-PRO system. Those who provide consent will be enrolled in the app-based e-PRO system on their smartphone. If the entered data are insufficient, enrolment will not be accepted until all fields are completed at baseline. The electronic data capture system will be used for data management and central monitoring. Because this exercise programme will not be invasive, there is no need to establish a data monitoring committee or to complete auditing in this study.
Random assignment and assignment adjustment factors
After enrolment and the additional input of the VO2peak value, patients will be randomly assigned. In the process of random assignment, VO2peak (obtained from the baseline measurement performed after obtaining informed consent) and age will be used as assignment adjustment factors, and automatic assignment will be performed using the data centre’s assignment feature. Only the measurer will be blinded in this study, because interventions differ for the intervention group and control group.
Dataset availability
The data sharing policy in this study is defined with reference to the example proposed by the International Committee of Medical Journal Editors: individual participant data will be made publicly available for a 5-year period through the University Hospital Medical Information Network—Individual Case Data Repository (https://www.umin.ac.jp/icdr/index-j.html).
Concomitant treatments
There are no regulated concomitant therapies or supportive therapies in this study.
Stopping rules for participants
Situations in which the intervention protocol is terminated for any of the following reasons will be defined as stopping of the protocol intervention: (1) dropping out due to withdrawal of consent or inability to measure the primary endpoint (VO2peak); (2) if a primary physician deems it necessary to stop the protocol intervention for a participant due to adverse events such as stress; (3) death of the patient during the protocol intervention period; (4) sudden worsening of the participant’s condition after enrolment or discovery of a protocol violation or ineligibility. A researcher will report the reason for stopping the protocol intervention to the data centre. In this event, as long as consent is not withdrawn, follow-up including the questionnaire survey will be continued.
Stopping of the assessment
Situations in which the participant declines assessment will effectively stop the assessment. A researcher will confirm the possibility of implementing the remaining parts of the intervention and follow-up with the participant. Stopping of the assessment will be recorded along with the details of the reason for stopping. As a rule, the remaining aspect of follow-up will be implemented as stipulated per protocol.
Assessment measures
Table 1 shows the outcome measurement schedule.
Primary outcome measure: cardiorespiratory fitness (VO2peak)
Schedule for outcome measurement
VO2peak is the maximum oxygen uptake observed during exercise tolerance testing and is used as an indicator of cardiorespiratory fitness.26 27 For each participant, VO2peak will be measured using the incremental multistage load method with a bicycle ergometer (Ergomedic 828E, Monark, Stockholm, Sweden) at 60 revolutions per min. The test will begin at 0.5 kp and increase by 0.25 kp per min until exhaustion. When the participant’s pedal rotation speed drops below 55 revolutions per min three times, the test will be deemed concluded. Respiratory gases will be analysed using an automatic gas analyzer (Air Monitor AE-310S, Minato Medical Science Co., Osaka, Japan). Rated perceived exertion and HR will be recorded after 45 s in each stage. The maximum value of VO2 observed during exercise will be used as the VO2peak.
Secondary outcome measures
Physical function
An alternative indicator of VO2peak, the 6 min walk test (m), will be performed.28 Walking speed (m/s), a diagnostic criterion for sarcopenia, will also be measured. A 5 m section in the middle of an 11 m line will be measured to calculate walking speed.29 One-repetition maximum for leg press, which reflects muscle strength in the lower body, will be assessed using a leg press machine (Powertec Leg Press P-LP16; Powertec, Paramount, California).30 31 After a thorough warm-up, measurements will be performed by incrementally increasing the load until a weight, which can be lifted only once, is reached. Load will be increased by two levels at a time. Grip strength (TKK 5401 Grip-D; Takei, Niigata, Japan) will also be measured.31 32 The chair stand test, which reflects combined leg strength for the lower limbs as a whole, will be performed.31 33 Participants will sit and stand from a chair for 10 s and the number of chair stands will be measured. The timed up and go test, which is used in clinical settings as an assessment of functionality as well as muscular strength, will also be performed.31 The functional reach test, which as a method of assessing dynamic balance is considered an indicator of expected fall risk, will also be performed.34 While keeping the hand at the same height, the participant will extend their arm forward as far as possible, without moving the feet, to make a mark at the farthest point they can reach. Lifting the heels and standing on tiptoe is allowed. The two-step test will also be performed.35 This test comprehensively assesses ability to walk, including leg strength, balance and flexibility. Based on bioelectrical impedance analysis, a body composition metre (MC-780A-N, TANITA, Japan) will be used to send a weak electrical current through the body via electrodes.
Physical activity level
The Global Physical Activity Questionnaire (GPAQ) was developed in 2002 as an internationally standardised questionnaire for surveying physical activity level. The GPAQ is widely used in policy development by WHO.36–38 The face validity of the Japanese version has been confirmed.39 In addition, as an objective measure of physical activity during the research period, resting HR, steps, distance, calorie expenditure and the duration of each sleep stage as well as wake up time will be measured using an activity monitor and logged for 24 hours periods (Fitbit versa, Fitbit). Maximum HR during exercise will also be measured to confirm whether the intensity of the exercise being implemented is appropriate. The accurately measured group will be defined as the group who wears the Fitbit versa at least 60% of the time during the 12-week intervention period.
Subjective measures
Fear of cancer recurrence (FCR) will be assessed by the overall fear index score on the Concerns About Recurrence Scale.40 41 This instrument comprises four items scored on a 6-point Likert scale (range, 1–6), with a higher score indicating worse FCR. Depression will be assessed using the Patient Health Questionnaire-9.42 Fatigue will be assessed by the Cancer Fatigue Scale. Sleep will be assessed by the Athens Insomnia Scale.43 44 Health-related QOL will be assessed using the EuroQol 5 Dimensions questionnaire.45 46
Biological assessments
To assess changes in gut microbiota, intestinal metabolites and intestinal immunity,47 a 1 g faecal sample will be obtained at baseline and at 12 weeks. Blood compositions of n-3 polyunsaturated fatty acids48 will be assessed from capillary dried blood spot samples (approximately 80 µL) at baseline.49
Medical economic costs
For cost–benefit analysis, the number of staff, working hours, labour costs, equipment costs, office expenses, number of unexpected medical consultations, direct medical costs and costs of other medical services used will be obtained.
Harms
The intervention in this research will potentially place stress on participants physically and in terms of their time, because it will take approximately 90 min. There will also be temporary exhaustion, although individual differences will be considered in exercise implementation. There are no financial risks associated with participation in the study.
Compensation
If participants develop unexpected health issues due to study participation during or after completion of the study, treatment will be provided appropriately in the same way as standard medical care. Medical expenses at that time will be handled within the medical insurance to which the participant is enrolled. No financial compensation, except for providing the wearable device, will be given in this study.
Data analysis
The primary endpoint, VO2peak, will be calculated as follows. Measurements will be taken at the start of the intervention (0 week) and at the conclusion of the intervention (12 weeks). The analysis set for primary analyses will consist of all randomised subjects. Our primary analysis is intention-to-treat analysis and patients without outcome data will be excluded from the analyses. After completing primary endpoint data locking, analyses centring on the primary endpoint will be performed. The objective of primary analysis in this study is to investigate whether the habit-B programme group (trial treatment group) surpasses the group receiving treatment as usual (control) in VO2peak, the primary endpoint. One-sided tests will be used, because, if the trial intervention group is inferior to the control group, it is not important in this trial whether that difference is statistically significant. The trial will adopt a one-sided significance level of 2.5%. A between-groups comparison will be performed using an independent two-sample t-test to determine the significance of amount of change in VO2peak (mL/kg/min) from 0 to 12 weeks (after intervention completion) between the trial intervention and the control groups. Secondary endpoint analyses will be performed with the goal of supplementing the primary analyses. The detailed methods for supplementary analyses will be specified in the Statistical Analysis Plan before the study data are fixed. Once all data have been locked following conclusion of the primary analysis period and follow-up, final analyses will be performed.
Sample size estimation
The main hypothesis of this study is that the intervention group will significantly surpass the control group in terms of the amount of change in VO2peak from 0 to 12 weeks. Regarding the clinical significance of the results of an exercise programme, it is common to use an increase of 10% in VO2peak to evaluate the effects of an exercise program.50 As such, an increase of 10% from VO2peak at the start of exercise has been established for this trial. According to a previous study of intervention similar to that in the present study,51 the SD for the VO2peak of the intervention group was 2.6 mL/kg/min. Based on the above hypothesis, by estimating the number of subjects required for a one-sided 2.5% and a power of 80% in the analysis, 28 individuals per group are necessary for a total of 56 in both groups (SAS V.9.4 software; SAS Institute, Cary, North Carolina). With the estimation that 4 participants will drop out, enrolment of 60 patients is planned.
Study period
The study period of this trial will be from April 2019 to March 2021; the participant entry period will be from April 2019 to March 2020. Study enrolment, intervention and data collection are ongoing.
Patient and public involvement statement
This study protocol was designed with the involvement of a breast cancer survivor who participated in this study as a researcher and coauthor. She discussed issues with other survivors in instances where survivors’ preferences and opinions should be considered. In the process of creating the habit-B programme, we conducted a preliminary confirmation of the feasibility and safety of this programme in five breast cancer survivors.
Discussion
An excellent review by Shapiro et al affirms the positive role that high levels of physical activity play in improving health-related QOL and symptom management (eg, chronic pain, fatigue, insomnia, sexual dysfunction, metabolic syndrome, bone loss, cognitive dysfunction and depression), and return to work in cancer survivors.52 In addition, increased cardiorespiratory fitness may decrease all-cause mortality among cancer survivors.53 54 However, there are currently no effective home-based exercise programme available for improving cardiorespiratory fitness for breast cancer survivors. No studies have precisely investigated cardiorespiratory fitness55; however, a small pilot study did estimate VO2 based on subjective exercise intensity among Japanese breast cancer survivors. Accordingly, the present study seeks to confirm whether our originally developed home-based exercise programme (habit-B programme) improves VO2peak compared with treatment as usual with wearable device in sedentary breast cancer survivors in Japan. With the assumption that the programme will be widely implemented if successful, it was designed to be (1) home based, (2) quick to implement (only 10 min in total), (3) use only body weight exercises involving the lower limbs and (4) use a wearable device for which personalised ICT support is available. We believe that no similar studies have been implemented.
If it is found that the habit-B programme is effective in increasing VO2peak, we will then proceed to the next trial, aiming for its widespread implementation in society. Specifically, the aims of the next study will be (1) to investigate whether intervention using the programme developed in this research but presented in a simpler format is effective in establishing exercise habits and (2) to assess whether the programme can be implemented in societies in Eastern Asia, including in both urban and rural Japan and other East Asian countries.
We strongly believe that a successful support team comprising specialists in the field of exercise science, medicine, rehabilitation, nursing and patient advocacy, as involved in this study, will provide a new horizon in cancer survivorship care.
Acknowledgments
The authors sincerely thank Hiroji Iwata, Hirokazu Arai, Noriko Watanabe, Noriaki Tatematsu, Hikaru Ihira, Tomoyasu Chigahara, Shinji Fukuda and Eiko Saito for their generous support for the study. They also thank Ayako Sato for her role as an exercise model.
References
Footnotes
KT and EO contributed equally.
Contributors KT, EO, RO, YS, TK and YM conceived the study and drafted the original protocol. KT, EO, RO, YS, TS, AK, TU, TK, and YM participated in refining the protocol. TU developed app and EDC system and managed datacenter. AK played a major role in the statistical analysis. KT, EO, TK and NS contributed to developing the exercise program. KT, EO and YM drafted the manuscript. All authors participated in, read, and approved the final manuscript.
Funding This study is supported by a National Cancer Center Research and Development Fund (30-A-17).
Competing interests EO has received research support from Nippon Suisan Kaisha. YM has received speaker fees from Suntory, Pfizer, Mochida, Eli Lilly, Morinaga Milk and NTT Data and is conducting collaborative research with Morinaga Milk and SUSMED. AK has received speaking fees from Chugai Pharmaceutical Co. All other authors declare that they have no competing interests regarding this work.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.