Article Text
Abstract
Introduction Cerebrovascular disease—such as stroke—is the second most common cause of dementia (ie, vascular dementia). Specifically, a stroke increases one’s risk for dementia by a factor of two. Thus, stroke survivors represent a target population in need of intervention strategies to promote cognitive function and prevent dementia. The current standard of care in stroke rehabilitation does not adequately address the significant cognitive consequences of stroke, especially for those who are in the chronic phase (ie, >12 months since an index stroke). Two potential intervention strategies are: (1) exercise training and (2) cognitive and social enrichment activities.
Methods and analysis The aim of this proof-of-concept randomised controlled trial is to determine whether a 6-month targeted exercise training programme or a 6-month cognitive and social enrichment programme can efficaciously and efficiently improve cognitive function in older adults with chronic stroke compared with a 6-month stretch and tone programme (ie, control). The primary measurement periods will be baseline, month 6 (postintervention) and month 12 (6-month follow-up). The primary outcome measure will be performance on the Alzheimer’s Disease Assessment Scale-Cognitive-Plus (ADAS-Cog-Plus), a global measure of cognitive performance using multidimensional item response theory to summarise scores from the 13-item ADAS-Cog and other standard cognitive assessments. The primary analysis will compare changes in ADAS-Cog-Plus performance from baseline to month 6. Proof-of-concept outcomes relating to intervention feasibility will be analysed descriptively. The economic evaluation will examine the incremental costs and health outcome benefits generated by both interventions versus the control.
Ethics and dissemination Ethical approval has been obtained from the University of British Columbia’s Clinical Research Ethics Board (H13-00715, 26 July 2013). Any modifications to the protocol will require a formal amendment to the protocol and approval by the Research Ethics Board. Outcomes of this randomised controlled trial and the statistical code to generate those outcomes will be disseminated through publication in peer-reviewed journals as well as conference presentations.
Trial registration number NCT01916486.
- chronic stroke
- cognitive function
- cognitive training
- exercise
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
First study to directly compare exercise training to cognitive training relative to a credible control condition among individuals with chronic stroke.
Randomised controlled trial comparing 6 months of exercise training or social and cognitive enrichment to an active control of balance-and-tone training in individuals who have experienced a stroke at least 12 months prior.
The primary outcome measure is general cognitive performance with secondary measures of executive functioning, mood, quality of life, sleep quality and cardiometabolic functioning.
Six-month follow-up assessment will determine whether any treatment effects persist.
The study is assessor blinded as it is not feasible to blind participants to treatment condition.
Introduction
One in six older adults will suffer a stroke in their lifetime, or one stroke every 2 s worldwide.1 Of relevance to our study, cerebrovascular disease—such as stroke—is the second most common cause of dementia (ie, vascular dementia),2–5 accounting for up to 38% of all dementia cases.6 Specifically, a stroke doubles one’s risk for dementia.7 Moreover, impairments in several domains of cognition—including memory, attention and executive function—are common following stroke.8–10 Stroke-related cognitive deficits are associated with other negative outcomes, including institutionalisation,11 reduced quality of life12 and death.13 Thus, stroke survivors represent a target population in need of intervention strategies to promote cognitive function and prevent dementia. However, the current standard of care in stroke rehabilitation does not adequately address the clinically important cognitive consequences of stroke—especially for those who are in the chronic phase (ie, >12 months since an index stroke).
Current evidence from randomised controlled trials (RCTs) suggests that targeted exercise training is an effective strategy to promote both cognitive and functional brain plasticity in older adults.14–20 A meta-analysis concluded that aerobic training has robust but selective benefits for cognitive function; the largest benefits occur for executive function.15 Rodent models have shown that exercise training induces upregulation of neurotrophic factors within the central nervous system that, in turn, contribute to neural health21 22 and myelin recovery following pathological insult.23 24
However, there is insufficient quality evidence for targeted exercise training as an effective strategy to promote cognitive function in stroke survivors.25 26 Despite the high prevalence of cognitive deficits and the increased risk for dementia in this population, few RCTs to date25 27 28 have focused on targeted exercise training on cognitive function. A small-scale RCT of individuals with chronic stroke (> 6 months postischaemic stroke) showed improved processing speed following 8 weeks of aerobic exercise training but no effects on other aspects of cognition compared with 8 weeks of stretching.28 A second small-scale RCT found that a 19-week multicomponent exercise training programme (adapted from the Fitness and Mobility Exercise (FAME) programme) improved general cognition and increased cerebral blood flow compared with a strength programme of equal length.29 A pre–post designed study showed that general cognition and executive function were improved following 6 months of combined aerobic and resistance training in individuals who had experienced a stroke at least 10 weeks prior30; however, the lack of a control group precludes causal conclusions. In contrast, a recent RCT did not find that 6 months of aerobic exercise significantly improved aspects of memory and executive function relative to low-intensity balance and tone training.31 In light of the promising evidence from previous pilot studies, from studies of older adults without chronic stroke,28 and from mechanistic animal studies,23 24 further research of exercise training among individuals with chronic stroke in large-scale, well-designed RCTs is needed.
Nevertheless, the physical ability of stroke survivors to participate in targeted exercise training is often limited. In fact, most stroke survivors adopt or return to sedentary lifestyles after rehabilitation.32 Poststroke physical deficits (eg, balance) are associated with reduced activity participation.33 Additional barriers to physical activity participation include low self-efficacy and social support.34 Thus, other strategies to promote cognitive function need to be considered for this population.
An alternative or supplemental behavioural approach might be to intervene with cognitive and social enrichment activities to ameliorate cognitive impairment in the chronic stroke phase. The premise of this strategy is that by engaging in activities that stimulate higher-order cognition (eg, memory and executive function), cognitive performance is improved and future cognitive decline is mitigated. Rodent models suggest that environment enrichment, for example, housing in larger cages and in larger groups with varied environmental features has various positive behavioural, neuroanatomical and molecular effects, including following stroke.35 Few previous studies have tested this proposition among humans with chronic stroke. A 6-month pre–post study showed that a programme that combined exercise training using the FAME programme (2 days/week) with cognitive and social enrichment (1 day/week) was associated with benefits in aspects of memory and executive functions among individuals who had sustained a stroke at least 12 months prior.27 Another pilot RCT compared this same multicomponent intervention to a wait-list control and found benefits to aspects of executive functions, working memory and physical functioning.36 Whether these effects could be attributed primarily to the exercise training or to the social and cognitive activities could not be determined by this study design.
Thus, to better understand the relative effects of exercise training and of cognitive and social enrichment on cognitive function, we aim to conduct a three-arm, parallel group proof-of-concept RCT comparing the following: (1) exercise training, (2) cognitive and social enrichment activities and (3) an active control group consisting of stretching and toning activities. Each intervention arm will be 6 months in length and will be followed by a 6-month follow-up period. The results of this proof-of-concept RCT will be used to inform the design of a larger definitive trial. Specifically, it will confirm the feasibility of the study methods and procedures.
Methods and analysis
Design outline
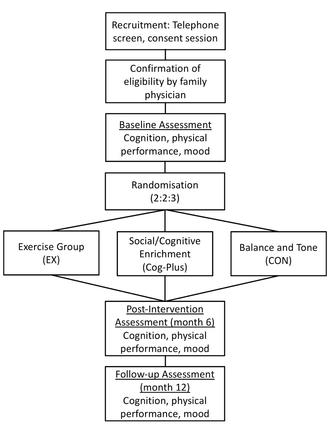
We will conduct a 6-month proof-of-concept RCT and follow up our study cohort for an additional 6 months (see figure 1). There will be a dedicated research coordinator (non-blinded) and trained assessor (blinded). Standardised protocols will be developed and study personnel will be trained by the research team. Assessments and intervention classes will occur at a research laboratory on the Vancouver General Hospital campus, Vancouver, Canada.
Overview of the flow of participants through from recruitment to study completion. Cog-Plus, Cognitive-Plus.
Recruitment
Recruitment advertisements will be placed in local community centres, stroke support groups and newspapers in Greater Vancouver. Interested individuals will initially be screened by telephone by the research coordinator using both the inclusion criteria and the modified Physical Activity Readiness Questionnaire (PAR-Q),37 a screening measure of physical readiness for exercise that requires clearance by a physician to engage in exercise. Those who appear eligible will be invited to an information session. During the information sessions, potential participants will be provided with details of the study and will have the opportunity to ask questions. A consent and screening session will be arranged for those who are interested in participating at the end of the information sessions. Those who remain eligible after the screening session will proceed to baseline assessments after their physician provides: (1) a written recommendation indicating their appropriateness to participate in an exercise programme and (2) a detailed description of their stroke (ie, when it occurred, lesion location and lesion type as defined by previous MRI or CT scans).
Time frame
Recruitment began in December of 2013 and the estimated completion date for collection of the primary outcome measure is December of 2018. To date, 72 individuals (~61% of target sample) have been randomised.
Eligibility
Inclusion criteria
Community-dwelling adults will be included who have had an ischaemic or haemorrhagic stroke (confirmed by previous MRI or CT scan). In addition, individuals must meet the following inclusion criteria: (1) are aged 55 years and over; (2) have a history of a single stroke of at least 1 year prior to study enrolment; (3) have a Mini-Mental State Examination (MMSE)38 score of > 20/30 at screening, including a perfect score on the three-step command to ensure intact comprehension and ability to follow instructions; (4) are community dwelling; (5) live in Greater Vancouver area; (6) able to comply with scheduled visits, treatment plan and other trial procedures; (7) read, write and speak English with acceptable visual and auditory acuity; (8) not expected to start or are stable on a fixed dose of cognitive medications (eg, donepezil, galantamine, etc) during the 12-month study period; (9) able to walk for a minimum of 6 m with rest intervals with or without assistive devices; (10) based on interview, have an activity tolerance of 60 min with rest intervals; (11) not currently participating in any regular therapy or progressive exercise and (12) provide a personally signed and dated informed consent document indicating that the individual (or a legally acceptable representative) has been informed of all pertinent aspects of the trial. In addition, an assent form will be provided at baseline and again at regular intervals.
Exclusion criteria
Individuals will be excluded who are: (1) diagnosed with dementia of any type; (2) diagnosed with another type of neurodegenerative or neurological condition (eg, Parkinson’s disease) that affects cognitive function and mobility; (2) at high risk for cardiac complications during exercise or unable to self-regulate activity or to understand recommended activity level (ie, Class C of the American Heart Risk Stratification Criteria); (3) have clinically important peripheral neuropathy or severe musculoskeletal or joint disease that impairs mobility, as determined by his/her family physician; (4) taking medications that may negatively affect cognitive function, such as anticholinergics, including agents with pronounced anticholinergic properties (eg, amitriptyline), major tranquillisers (ie, typical and atypical antipsychotics) and anticonvulsants (eg, gabapentin, valproic acid, etc) or (5) aphasia as judged by an inability to communicate by phone.
Measurement
There will be three primary measurement sessions: baseline, 6 months and 12 months. Baseline measurements will be obtained prior to randomisation. Additional secondary measures will be assessed monthly by non-blinded assessors throughout the 12-month study. Data will be entered and scored using standard scoring procedures for each measure. Paper files will be held in secure filing cabinets, and digital data will be stored on encrypted hard drives in laboratory areas with limited key card access. All participant materials will be identified by identification number to maintain participant confidentiality.
Screening and consent session
For the screening and consent session, the study coordinator will readminister the PAR-Q,37 a screening measure of physical readiness for exercise. Global cognitive function will be assessed using the MMSE38 and the Montreal Cognitive Assessment.39 Eligible participants will be provided a form to be completed by their family physician to confirm the inclusion/exclusion criteria.
Descriptors and relevant covariates
At baseline, general health, demographics, socioeconomic status and education will be ascertained by a questionnaire. We will also document each participant’s American Stroke Classification,40 medication history and type (eg, ischaemic, haemorrhage), location (eg, middle cerebral artery) and structure (eg, posterior parietal cortex) of stroke from medical records/family physician. At each of the three primary measurement sessions, we will measure age in years, standing height in centimetres and mass in kilograms. We will assess activity of daily living using the self-report Functional Independence Measure.41 Participants will complete the Functional Comorbidity Index to estimate the degree of comorbidity associated with physical functioning.42
Primary outcome measures
Our primary measure of cognitive function will be the Alzheimer’s Disease Assessment Scale-Cognitive-Plus (ADAS-Cog-Plus). The ADAS-Cog-Plus score is computed using a custom script43 using the package ‘mirt’ in the statistical package R (www.r-project.org). The ADAS-Cog-Plus uses a multidimensional item response theory model to generate a global cognitive functioning score and SE of measurement for that score from the items of the ADAS-Cog and other standard cognitive assessments. For the current study, we used the 13-item ADAS-Cog,44 Trail Making Test Parts A and B,45 Digit Span Forward and Backward,46 and Animal and Vegetable Fluency46 as the input variables into the scoring algorithm. The scoring algorithm references data from the Alzheimer’s Disease Neuroimaging Initiative sample, which was composed of approximately 50% mild cognitive impairment (MCI) cases, 25% cognitively normal individuals and 25% dementia cases. Lower scores represent better cognitive performance; specifically, ADAS-Cog-Plus scores of approximately −1.0 indicate healthy cognitive functioning, of 0.0 indicate MCI and of 1.0 indicate dementia.43
Secondary outcome measures
Executive function
A computerised version of the Stroop task47 will assess the response inhibition and selective attention components of executive function. The task will be completed using the program E-prime using a Windows-based computer and Cedrus RB-540 response pad. Colour (eg, RED, BLUE) and non-colour (eg, DISK, SCREEN) words will appear individually on the screen with 2000 ms duration and will be printed in one of three colours (blue, green or yellow). Participants are instructed to press the response pad button that is the same colour as the font colour of the word as quickly and accurately as possible. Following 18 practice trials, the task consists of 42 neutral trials (eg, the word DISK printed in green font), 42 congruent trials (eg, the word GREEN printed in green font) and 42 incongruent trials (eg, the word GREEN printed in blue font) presented in random order. The outcome is the median response time for incongruent trials minus the median response time for congruent trials, using only trials with correct responses. Higher scores are indicative a stronger Stroop effect, and thus, poorer executive function.
Instrumental activities of daily living
Instrumental activities of daily living (IADLs) will be assessed using the self-report Lawton and Brody48 IADLs Scale. This scale subjectively assesses ability to telephone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medication and ability to handle finances.
General balance and mobility
We will use the Short Physical Performance Battery49 to assess general mobility and balance. For the Short Physical Performance Battery, participants are assessed on performances of standing balance, walking and sit to stand. Each component is rated out of 4 points, for a maximum of 12 points; a score <9/12 predicts subsequent disability.49 We will also measure knee extension (quadriceps) strength using the method employed by the physiological profile assessment50 and grip strength (in kg) using a digital Jamar isometric hand dynamometer.
Mood
Depression is a prevalent clinical entity in stroke survivors—it has been reported to be as high as 38%51—and is negatively associated with cognitive function.52 We will use the Center for Epidemiological Studies Depression Scale53 to assess for depression, which asks participants to respond by indicating the frequency of 20 items. High scores indicate greater depressive symptoms.
Quality of life
We will use the EuroQual 5-Dimentions 3-Levels (EQ-5D-3L)54 to assess health-related quality of life. The reliability and validity of the EQ-5D-3L in the stroke population have been established.55 Participants indicate the number of problems within the following five domains: mobility, self-care, usual activities, pain and anxiety/depression. A health state utility value is calculated from the scores on each of the five domains. Lower scores indicate poorer health state. Scores lower than zero indicate a health state considered worse than death.
Healthcare resource usage
Participants will complete monthly healthcare resource use diaries over the 6-month study period and use this information to respond to a healthcare resource usage questionnaire administered at 3 and 6 months.
Objective sleep quality
We will use the MotionWatch 8 actigraphy system (MW8; camntech) a light-weight, water-resistant, tri-axial wrist-worn accelerometer. The MW8 provides reliable, previously validated estimates of daytime activity and sleep quality including sleep duration (ie, total time asleep), efficiency (ie, actual sleep time expressed as a percentage of time in bed) and fragmentation (ie, a measure of sleep disruption during the sleep window).56 57 Participants will be fitted with the MW8 and provided detailed information on its features (ie, the light sensor, event marker button and status indicator). Participants will be instructed to press the event marker button each night when they started trying to sleep; and again each morning when they finished trying to sleep. Participants also will be given consensus sleep diary and asked to complete it on awakening each morning. We will record sleep quality with the MW8 and sleep diary over 14 days.
Blood biomarkers
For those who decide to participate and consent to an Optional Blood Draw for Biomarkers Subject Information and Consent Form, a blood draw will be conducted at Vancouver General Hospital looking at changes in lipid profile and insulin sensitivity.
Monthly measurement of secondary outcome measures
Current physical activity level
Current level of physical activity will be determined by the valid and reliable Community Health Activities Model Program for Seniors questionnaire.58 This 41-item questionnaire assesses participation in various activities, including physical activities of different intensities, for the previous 4 weeks. A metabolic equivalent is assigned to each activity. Participants will be asked to only report physical activity participation outside the research study.
Leisure activity level
Participation in leisure activity (eg, hobbies, volunteering, etc) will be determined by the Nottingham Leisure Questionnaire.59 The validity and reliability of this questionnaire in the stroke population have been established.59 Participants will be asked to only report leisure activity participation outside the research study.
Proof-of-concept outcome measures
Feasibility outcomes for delivering the intervention (ie, adherence) will be measured throughout the 6-month intervention period. Class attendance will be recorded by the instructors.
Treatment allocation and concealment
After patients have signed informed consent to agree to be involved in the trial, they will be stratified into two groups by stroke status (1 vs ≥2 prior stroke events) and then randomly allocated with an allocation ratio of 2:2:3 (EX:Cog-Plus:CON, respectively) using permuted blocks (size intentionally withheld) within each stratum. For random number generation, each stratum will have its own seed using Minitab, a statistical package to generate uniform random integers, to create the allocation order within each block. The statistician (CHG) will hold the randomisation book and will give out the allocations of individual patients one at a time to the three groups, and so these allocations will be concealed from patients, all study personnel and the investigators, except CHG, until the interventions are implemented. The specific blocks will be revealed for use in the statistical analyses once the database has been cleaned and is ready for the statistical analyses. This process will allow the blocking restriction to be considered in the data analyses along with the integrity of the randomisation.
Interventions
All exercise-based classes will be led by instructors who have formal expertise in delivering group exercise programmes to older adults. All classes will be 60 min in duration. All classes will have a maximum participant to instructor ratio of 4:1. Class attendance will be recorded by the instructors. To minimise contamination, only one class will occur at any given time in the same facility. In addition, there will be a minimum of 30 min between classes at any given facility. All intervention groups will include two times per week classes of 60 min each over 26 weeks. Fidelity across instructors and across time will be ensured by providing instructors with detailed protocols including pictures; regular observation and intervention classes by study principal investigator and coordinator and auditing with standard checklist to ensure intervention content is delivered accurately and consistently; and videotaping classes from each intervention arm across time.
EX group
The EX programme is a multicomponent intervention based in part on the FAME programme.60 We have developed specific guidelines and increments for each exercise in this programme to provide safe and objective progression of the participants. Participants will be familiarised with the 16-point Borg Rating of Perceived Exertion (RPE) and the scale will be visible in the room. We have previously used the RPE in individuals with chronic stroke and found it representative of myocardial exertion.61 Each class will have a 10 min warm-up, three core components—strength training (20 min); aerobic/agility training (20 min); and balance training (5 min)—and will end with a 5 min cool down. Strength training will consist of calf raises, squats, bicep curls, tricep extensions and an alternating fifth activity of either sliding back lunges or standing leg abduction. Exercises will be progressed by adding weight (eg, dumbbells, completing single calf raise vs double calf raise) or altering movement tempo (eg, fast concentric motion followed by slow eccentric motion, adding hold at bottom of squat). Aerobic/agility exercises will include heal and toe tapping, low and high knee marches, stepper exercise, agility ladders and figure 8 walking. Initially, participants will be asked to complete the exercise at an intensity corresponding to a RPE of 12. Exercise intensity will be progressed at a rate of approximately 1 RPE/month, with a final target RPE of 16 during month 6. Balance exercises will have participants complete various movements (eg, hit balloons, throw ball against wall, walk forward and backward, close eyes, rotate trunk, move arms) while standing and with feet in either side-by-side, semitandem or fully tandem positions. Heart rate monitors (Polar RS400) will be worn throughout class, with measurement occurring before class, at least two times during class and at the end of class. The Borg RPE will be administered at least two times during class and at the end of class. Exercise difficulty (eg, added weight, stepper height) will be recorded during each class, and the Timed-Up-and-Go task and Short Physical Performance Battery will be completed monthly to provide objective performance tracking.
Cog-Plus group
We have designed this programme based on the feedback received from our pilot study27 and based on current evidence.62 In addition, we aimed to design a programme that could feasibly be implemented in the community with minimal resources. Because impairment in multiple cognitive domains is common following stroke,8–10 the cognitive exercises included in the programme targeted various aspects of cognition, including learning and memory, processing speed, attention, working memory, and executive functions. Each class will begin with the participants being asked to memorise a seven-item word list. Next, each participant will complete approximately 15 min of the brain training programme Lumosity using an individually issued Apple iPad. Lumosity consists of various short games (typically 1–5 min) that target various aspects of cognition (eg, working memory, divided attention, processing speed). In each class, participants will be encouraged to complete at least five distinct games. For the remaining class time (approximately 30 min), the participants will complete a variety of social games and mental activities in pairs or as entire class. Some of these activities will use apps on the Apple iPads (eg, Heads-Up, Teledoodle), and others are based on improvisation and mental activities from the PERK program.63 At the end of class, participants will recall as many of the words from the word list; they will use the Notes program on the iPad to record the recalled words. Approximately every month, the class instructor will meet individually with the participants to show performance progress on the Lumosity training program and to discuss outstanding concerns and areas of improvement (eg, short-term memory, speed of responding). Every month, the 7-item word list will be replaced with a 15-item word list, and participants will be requested to recall those words immediately, as well as at the end of the class.
CON group
The CON programme will follow the protocol used in Dr Liu-Ambrose et al’s previous RCT.64 The CON protocol will consist of stretches, deep breathing and relaxation techniques, general posture education, general core control exercises, grip strength and dexterity exercises, and light isometric toning exercises. Some exercises from the EX programme will also be included but in a simplified format without progression (eg, double calf raises, heal and toe tapping, balance exercises). Once a month, the class will consist of educational lecture and will include topics such as sleep hygiene, goal setting and nutrition. This group will serve to control for confounding variables such as physical training received by travelling to the community centre for two times per week classes and changes in lifestyle secondary to study participation. The Timed-Up-and-Go task65 will be completed on a monthly basis to allow for objective physical performance tracking.
Data and adverse events monitoring
A Data and Safety Monitoring Committee will be established by coinvestigators who will be independent from the day-to-day conduct of the study and from the study funders. RH, JCD, LEM and CHG will review all adverse events reported in the study on a monthly basis. They will stop the study if the adverse events data demonstrate any hazards that are the result of the intervention. They will also ensure data sharing and fidelity. Data provided to project team members will exclude identifying participant information.
Strategies to promote adherence
We will implement strategies to promote adherence during the 6-month intervention as recommended by the literature.66–70 These will include: (1) monthly phone calls by the non-blinded research coordinators to encourage adherence to classes; multiple contacts have been shown to be more effective than single exposures66; (2) discussing participant barriers and developing coping plans and action plans67; (3) setting implementation intentions and concrete plans68 and (4) encourage participants to continually self-monitor their progress with monthly calendars provided by the study. This strategy has been identified as the most successful behavioural/cognitive approach when compared with all other current adherence techniques.69
Sample size calculation
We have designed our trial to allow the evaluation of statistical significance of the treatment effect between groups on the ADAS-Cog-Plus. A number of pharmaceutical RCTs in vascular dementia71–74—a population highly relevant to our proposed study—have shown positive cognitive effects as measured by the ADAS-Cog, and it has been suggested that the ADAS-Cog-Plus shows greater sensitivity to underlying changes in cognition.43 A previous RCT of physical activity in older adults at risk for Alzheimer’s disease with ADAS-Cog as the primary outcome measure demonstrated a standardised effect size of 0.60.17 We used interim results from one of our previous studies75—an exercise RCT in adults with mild subcortical ischaemic vascular cognitive impairment—for our sample size estimation. Based on data collected from 15 participants who have completed the RCT, we found the mean change in the ADAS-Cog score was 2.7 (SD=2.3) and 0.87 (SD=3.4) for the exercise training group and the control group, respectively. The minimally clinically relevant change (MCRC) on the ADAS-Cog varies between 3 and 5 points, with a change of > 4 being recommended by the Food and Drug Administration.76 Recently, Schrag and Schott77 established the MCRC empirically using data collected from the Alzheimer’s Disease Neuroimaging Initiative. They found that a 3-point change on the ADAS-Cog is an appropriate MCRC. Assuming a mean change of 3 points on the ADAS-Cog for both the EX and Cog-Plus groups and a mean change of 1 point for the CON group at 6 months, a common SD of 2.85, and an alpha of 0.05, 39 participants per group (ie, total sample of 117) will provide a power greater than 0.80.78
Statistical analyses
Primary outcome
This analysis will follow the intention-to-treat principal, such that all randomised participants will be included to estimate treatment effects, irrespective of deviations from treatment protocol (eg, loss to follow-up, non-compliance). This will be done using linear mixed models using maximum likelihood estimation. The model will include random intercepts and slopes, and fixed effects of time (baseline, month 6, month 12), treatment assignment (CON, EX, Cog-Plus) and their interaction. Baseline MMSE score will also be included as a fixed-effect covariate. Time will be specified as a categorical variable, thus allowing us to examine treatment differences at the primary endpoint (month 6) and then, as a secondary objective, whether those differences persist at the 6-month follow-up (month 12). Two planned simple contrasts will be performed using the Dunnett test.79 These contrasts will be employed to assess differences between: (1) the EX group and the CON group and (2) the Cog-Plus group and the CON group. The overall alpha will be set at 0.05. A secondary complete-case analysis will be conducted using this linear mixed model, in which participants with valid data at all time points will be included. As an exploratory strategy, multiple imputation will be used to judge the impact of missingness on the conclusions drawn from this study.80
Secondary and tertiary outcomes
Analyses will be descriptive; no alpha has been allocated. Point and interval estimates for the effect of the intervention on each of the secondary outcomes at 6 and 12 months will be determined separately using linear mixed models. Multiple linear regression analyses will also be performed to explore the association between change in cognitive function, after accounting for experimental group, baseline age, baseline global cognition and: (1) superior treatment adherence; (2) change in physical activity levels outside the research protocol and (3) change in general balance and mobility. Randomisation integrity will be determined by examining bias in the blocking sequence used to produce allocation sequence.
Economic evaluation: a cost–utility analysis
Our economic evaluation will examine the incremental costs and effects generated by using a (1) 6-month targeted exercise training programme or a (2) 6-month cognitive and social enrichment programme among older adults with chronic stroke compared with a 6-month stretch and tone programme (ie, control; comparator). The outcome of our cost-effectiveness analysis is the incremental cost–utility ratio (ICUR). By definition, an ICUR is the difference between the mean costs of providing the competing intervention divided by the incremental difference in QALYs, where ICUR=∆ Cost/∆ QALY.81 82 QALYs are calculated based on the quality of life of a patient (measured using health state utility values estimated from the EQ-5D-3L) in a given health state and the time spent in that health state. For any missing data, we will use a combination of imputation and bootstrapping to quantify uncertainty due to missing values.83 84
Proof-of-concept outcomes
Feasibility outcomes—such as recruitment rate, withdrawal rate, adherence and number of adverse events—will be treated as binary, with ‘success indicating the protocol is sufficiently robust to move forward with the large RCT with only small or no adaptation required, and ‘revise’ indicating a need for more substantive change before proceeding.85
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
Footnotes
Contributors JRB contributed to intervention development and prepared the first draft of the manuscript. JJE co-conceived the idea for the trial design and contributed to intervention development. JCD contributed to intervention development and economic analysis. RH, PAH, LEM and PG contributed to trial design and intervention development. CHG contributed to trial design and statistical analysis protocol, and created randomisation protocol. JCD, RH, LEM and CHG serve on the data and safety monitoring committee. TL-A co-conceived the idea for the trial, obtained grant funding and contributed to study and intervention design. All authors critically reviewed the manuscript and approved the final version.
Funding This work is being supported by the Jack Brown and Family Alzheimer Research Foundation Society and an operating grant (F12-03501) from the Canadian Institutes of Health Research to TLA.
Competing interests None declared.
Patient consent Obtained.
Ethics approval University of British Columbia’s Clinical Research Ethics Board.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.